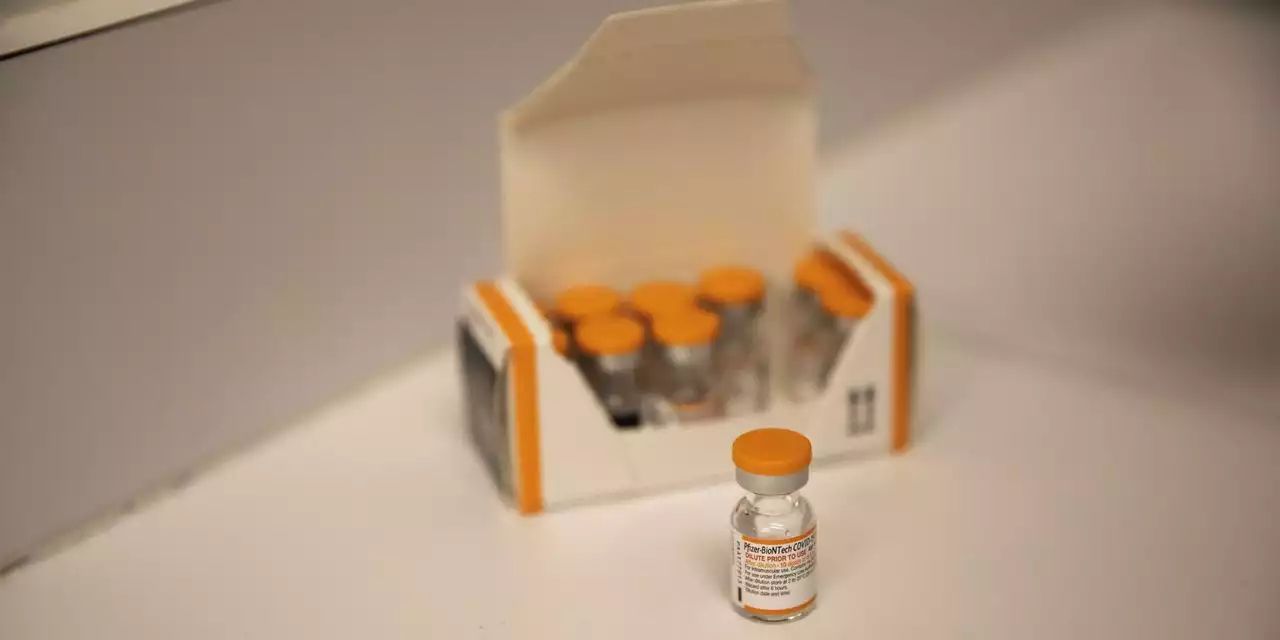
Vaccine advisers to the CDC are set to decide whether to recommend Covid-19 shots for children as young as 6 months
Two-day discussion ending Saturday marks step toward making Pfizer and Moderna shots available to one of last groups lacking access
Children under five in the U.S. aren’t yet eligible for a Covid-19 vaccine after efforts to get the shots to market have hit setbacks. WSJ’s Peter Loftus explains what we know about the vaccines and when they might become available.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
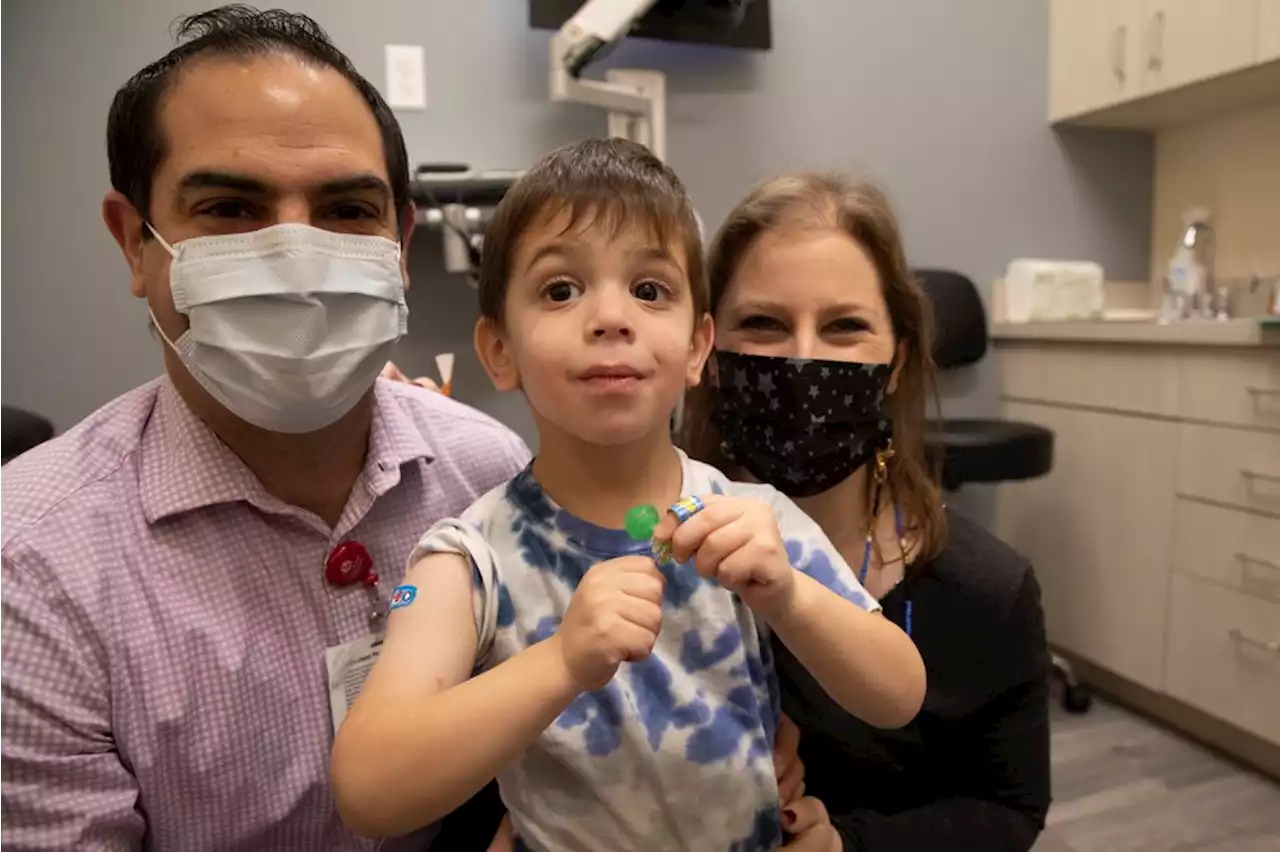 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Read more »
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Read more »
 FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
Read more »
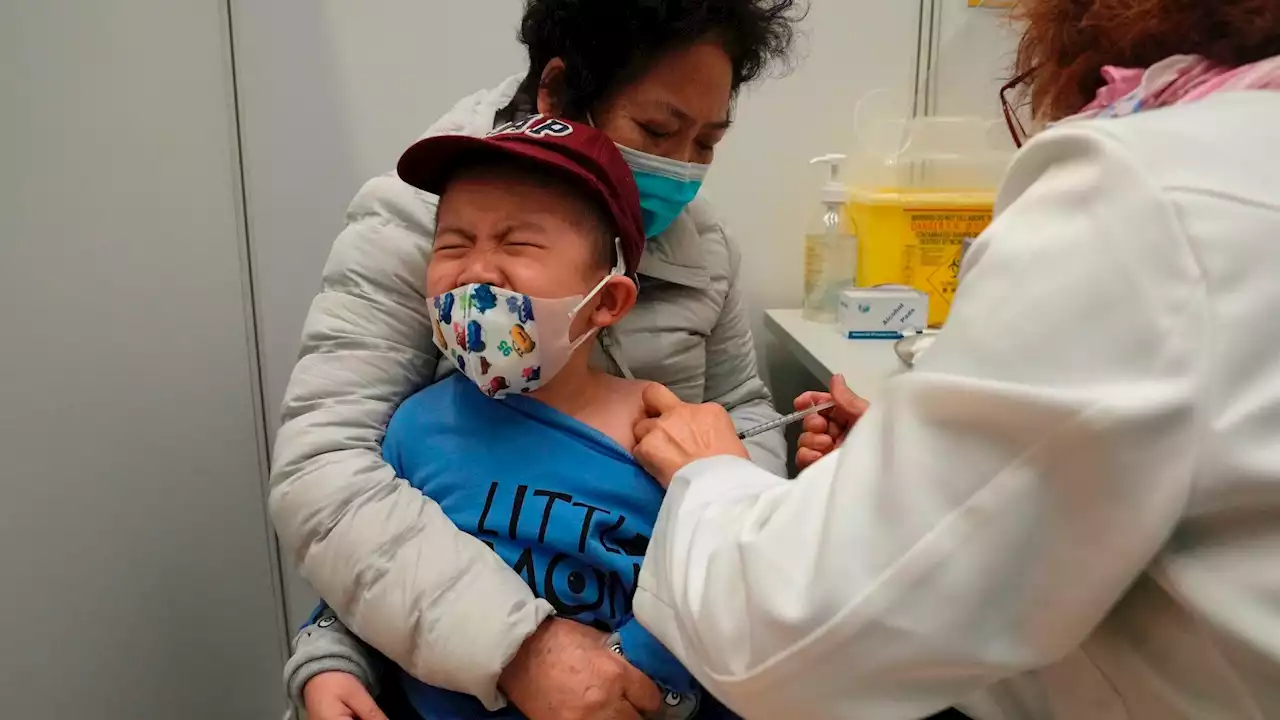 FDA Advisers Move COVID-19 Shots Closer for Kids Under 5The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
FDA Advisers Move COVID-19 Shots Closer for Kids Under 5The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
Read more »
 FDA advisers endorse 1st COVID-19 shots for kids under 5The first COVID-19 shots for infants, toddlers and preschoolers in the U.S. have moved a step closer
FDA advisers endorse 1st COVID-19 shots for kids under 5The first COVID-19 shots for infants, toddlers and preschoolers in the U.S. have moved a step closer
Read more »
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Read more »