U.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA reviewers said both brands appear to be safe and effective for children as young as 6 months old in analyses posted ahead of the all-day meeting. Side effects, including fever and fatigue, were generally minor in both, and less common than seen in adults.
An analysis from the U.S. Food and Drug Administration states that kid-sized doses of Pfizer's COVID-19 vaccine appears safe and effective for children under five, which would be a huge step towards vaccinating the nation's youngest children who were previously unprotected. Moderna’s shots are one-quarter the dose of the company’s adult shots. Two doses appeared strong enough to prevent severe infections but only about 40% to 50% effective at preventing milder infections. Moderna has added a booster to its study.
Pfizer’s submitted data found no safety concerns and suggested that three shots were 80% effective in preventing symptomatic coronavirus infections. But that was based on just 10 COVID-19 cases; the calculation could change as more cases occur in the company’s ongoing studies. The nation’s vaccination campaign started in December 2020 with the rollout of adult vaccines from Pfizer and Moderna, with health care workers and nursing home residents first in line. Teens and school-age children were added last year.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
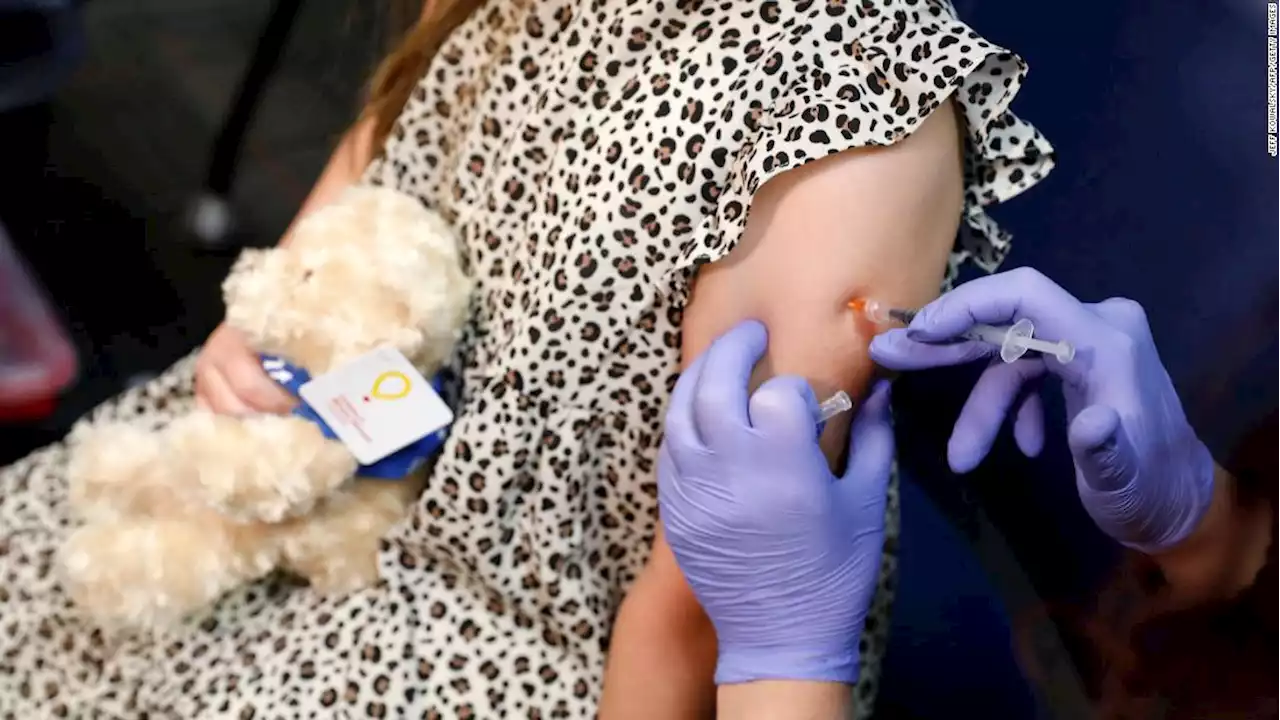 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
Read more »
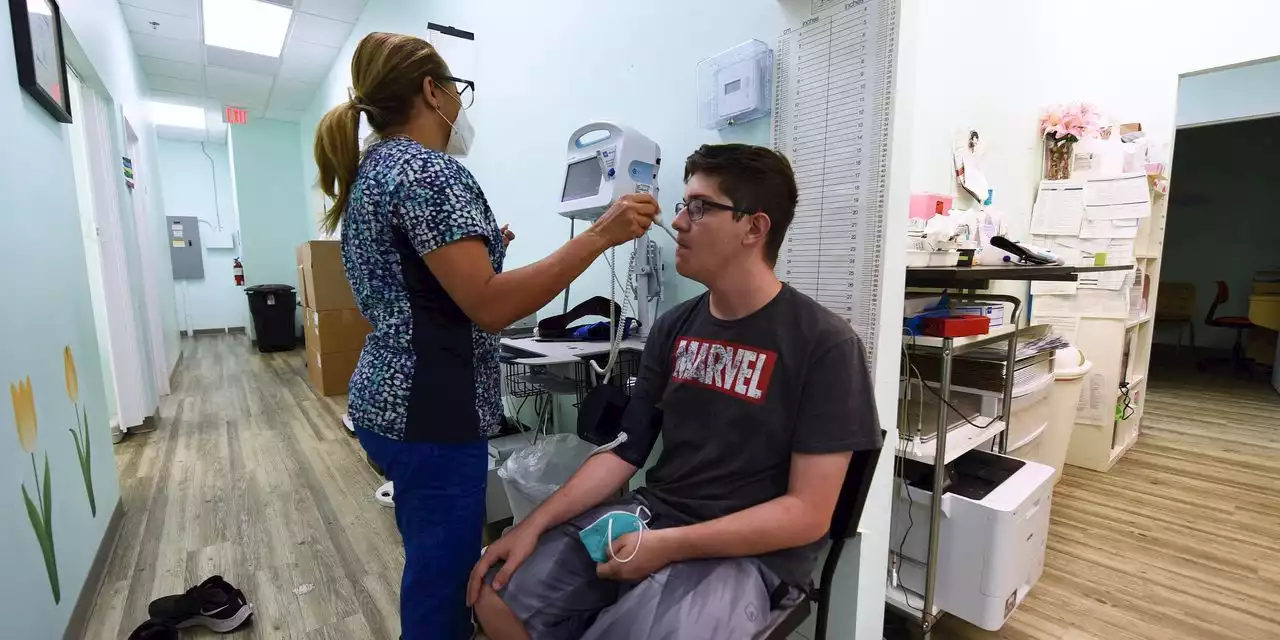 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
Read more »
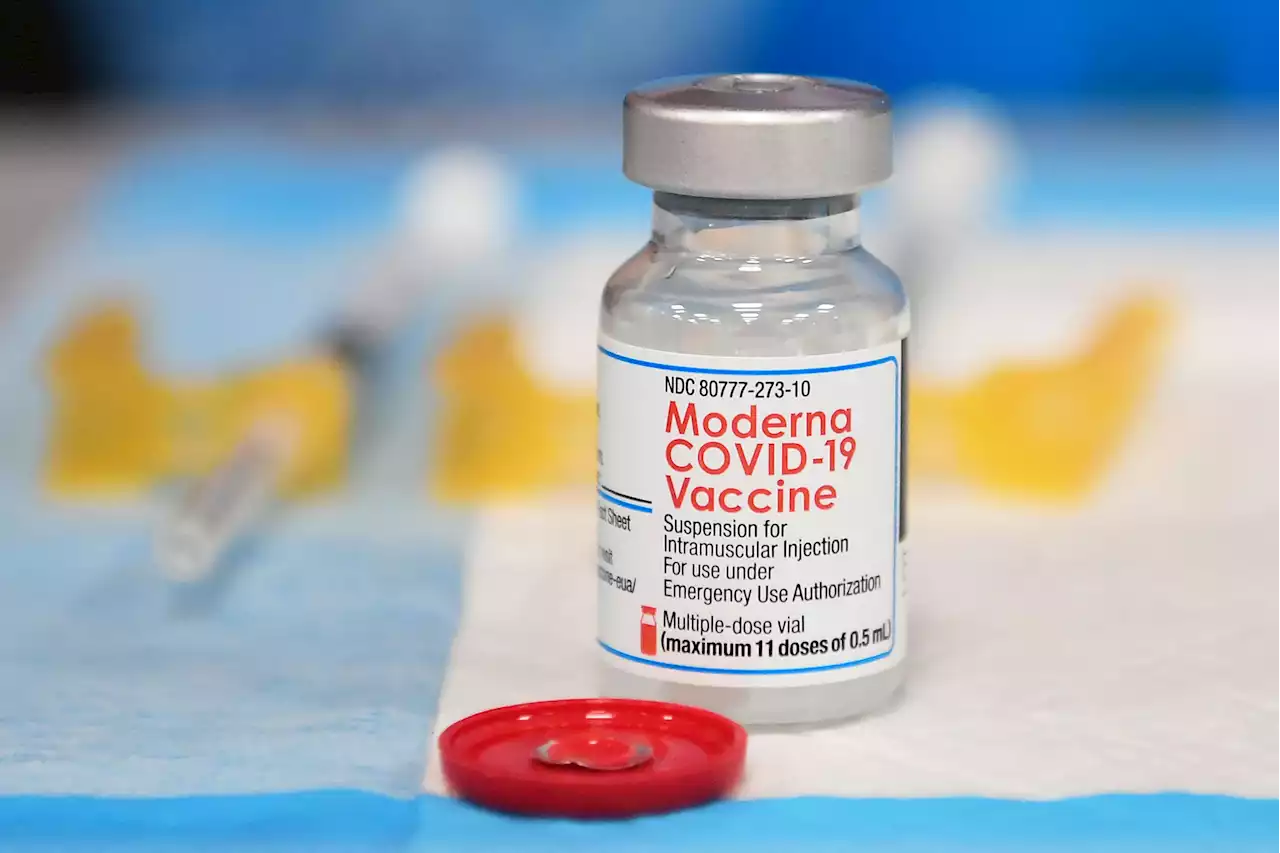 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
Read more »
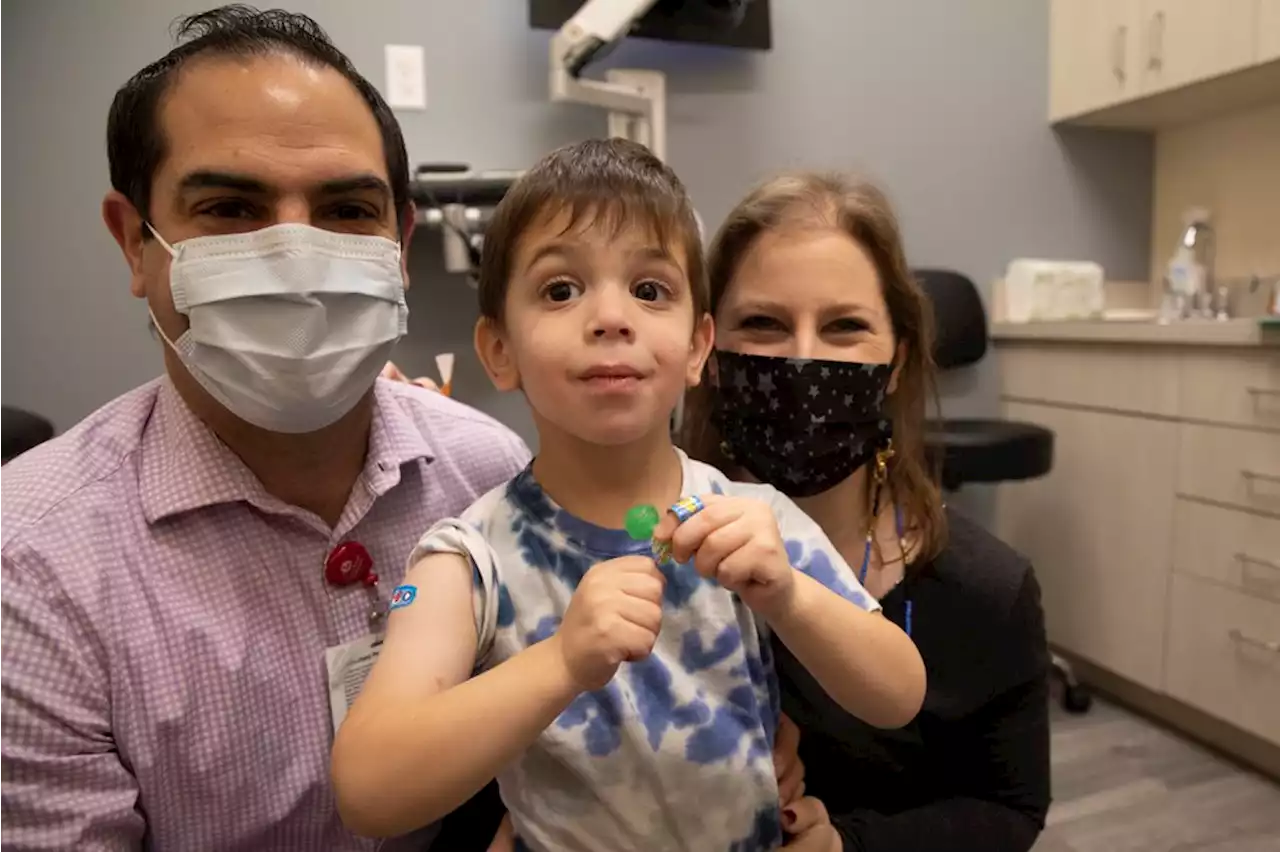 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Read more »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Read more »
 FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
Read more »
