A government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
In their review, FDA scientists said there were no confirmed cases of the heart inflammation in Moderna's kid studies. But experts say the studies may have had too few participants for a rare side effect like that to appear.
As for other side effects, FDA officials said nothing worrisome was reported — mainly sore arms, headache and fatigue. Vaccine effectiveness was estimated at 93% for the teens, and 77% for the younger children, according to the FDA analysis. However, the research was done when earlier versions of the coronavirus were causing most U.S. infections, and it's not clear how well they work against recent more contagious variants. It's also based on a limited number of COVID-19 cases, making the estimates a bit rough.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA says Pfizer's COVID-19 vaccine appears effective for kids under 5Federal health officials said Sunday that kid-sizes doses of Pfizer’s COVID-19 vaccines appear to be safe and effective for kids under 5. If regulators clear the shots, vaccinations could begin as soon as next week.
FDA says Pfizer's COVID-19 vaccine appears effective for kids under 5Federal health officials said Sunday that kid-sizes doses of Pfizer’s COVID-19 vaccines appear to be safe and effective for kids under 5. If regulators clear the shots, vaccinations could begin as soon as next week.
Read more »
 FDA says Pfizer COVID-19 vaccine appears effective for children under 5The FDA said Pfizer's coronavirus vaccine appears to be safe for children younger than 5, the only age group not yet eligible for vaccination in the U.S.
FDA says Pfizer COVID-19 vaccine appears effective for children under 5The FDA said Pfizer's coronavirus vaccine appears to be safe for children younger than 5, the only age group not yet eligible for vaccination in the U.S.
Read more »
 FDA: Pfizer COVID-19 Shot Appears Effective For Kids Under 5The review by federal health officials was a key step toward a long-awaited decision to begin vaccinating the youngest American children.
FDA: Pfizer COVID-19 Shot Appears Effective For Kids Under 5The review by federal health officials was a key step toward a long-awaited decision to begin vaccinating the youngest American children.
Read more »
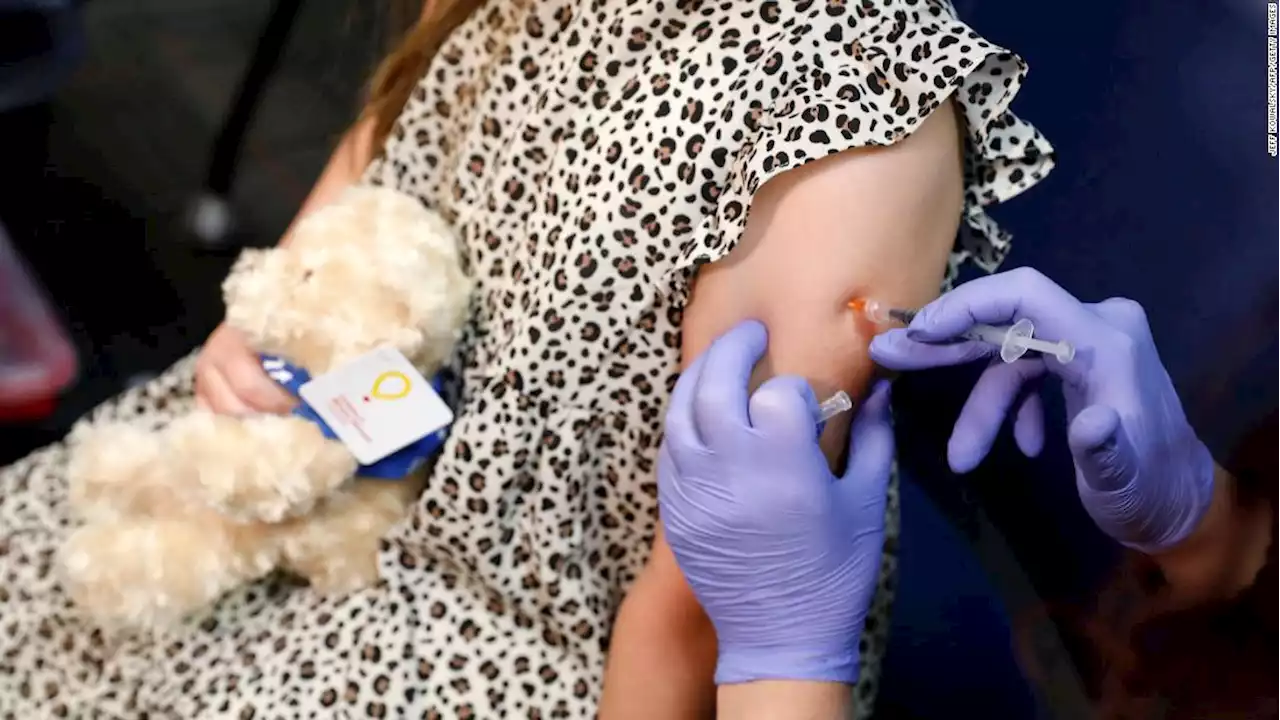 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
Read more »
 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
Read more »
 US: Pfizer COVID-19 appears effective for kids under 5A review by federal health officials says that Pfizer's COVID-19 vaccine appears safe and effective for children under 5, the only group not currently eligible for vaccination.
US: Pfizer COVID-19 appears effective for kids under 5A review by federal health officials says that Pfizer's COVID-19 vaccine appears safe and effective for children under 5, the only group not currently eligible for vaccination.
Read more »
