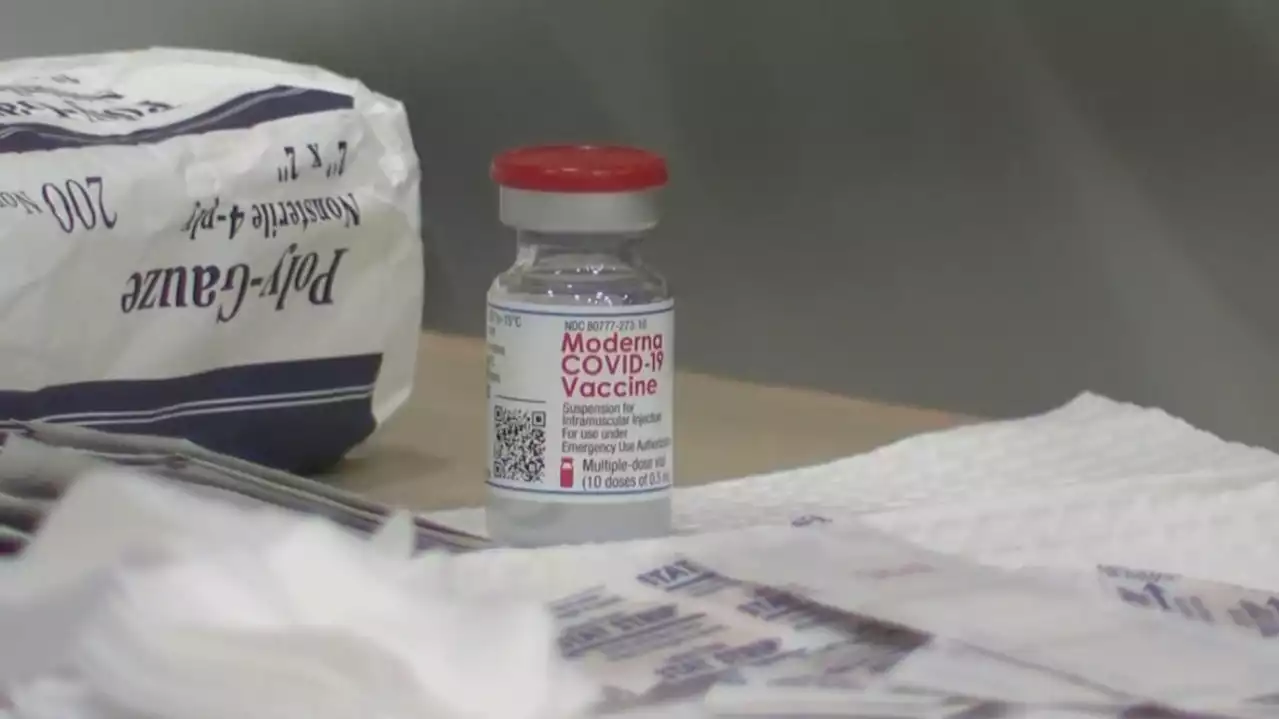
JUST IN: The FDA's outside vaccine advisers give thumps-up to Moderna's COVID-19 vaccine for children under 5.
PHILADELPHIA — COVID-19 shots for U.S. infants, toddlers and preschoolers moved a step closer Wednesday. The Food and Drug Administration’s outside vaccine advisers gave a thumbs-up to Moderna’s two shots for the littlest kids. The panel is set to vote later Wednesday on whether to also recommend Pfizer’s three-shot series for those youngsters.
“This is a long-awaited vaccine,” said one panel member, Dr. Jay Portnoy of Children’s Hospital in Kansas City, Missouri. “There are so many parents who are absolutely desperate to get this vaccine and I think we owe it to them to give them a choice to have the vaccine if they want to.” “That is a really important point,”′ said Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief. “You can’t compare the vaccines directly.”
Moderna’s shots are one-quarter the dose of the company’s adult shots. Two doses appeared strong enough to prevent severe illness but only about 40% to 50% effective at preventing milder infections. Moderna has added a booster to its study and expects to eventually offer one. The same FDA panel on Tuesday backed Moderna’s half-sized shots for ages 6 to 11 and full-sized doses for teens. If authorized by the FDA, it would be the second option for those age groups. Currently Pfizer vaccine is their only choice.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
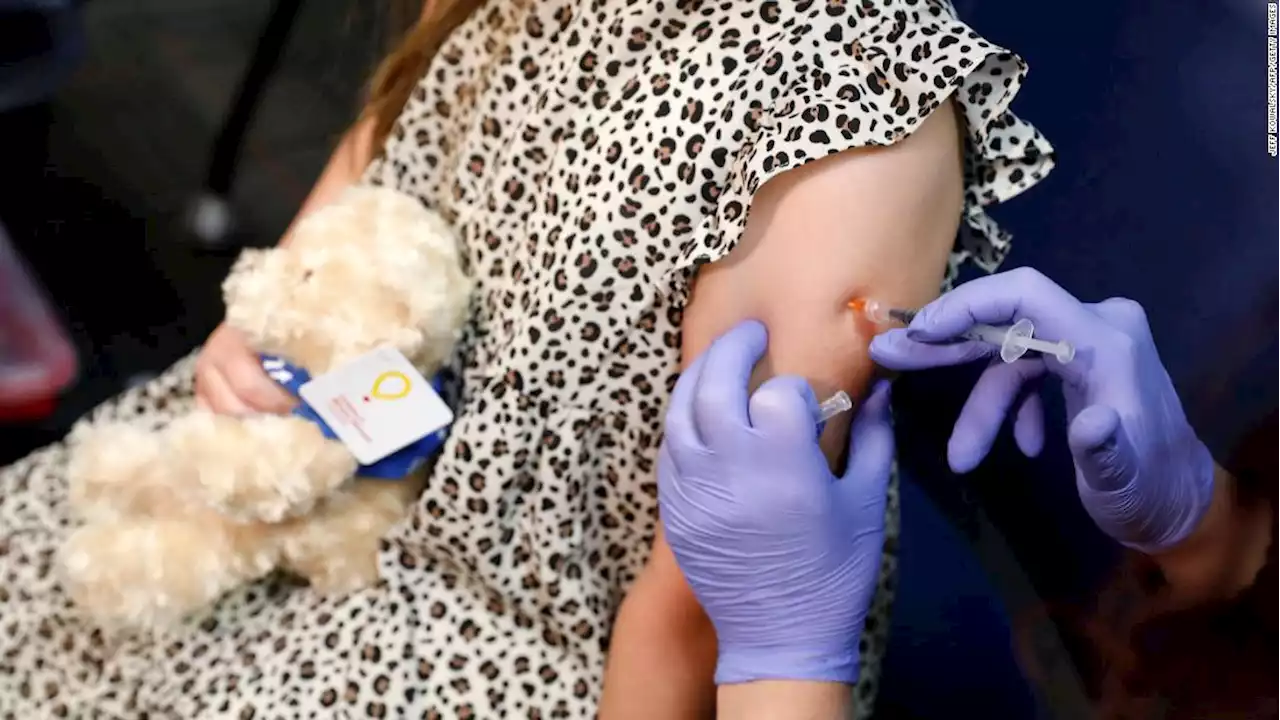 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
Read more »
 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
Read more »
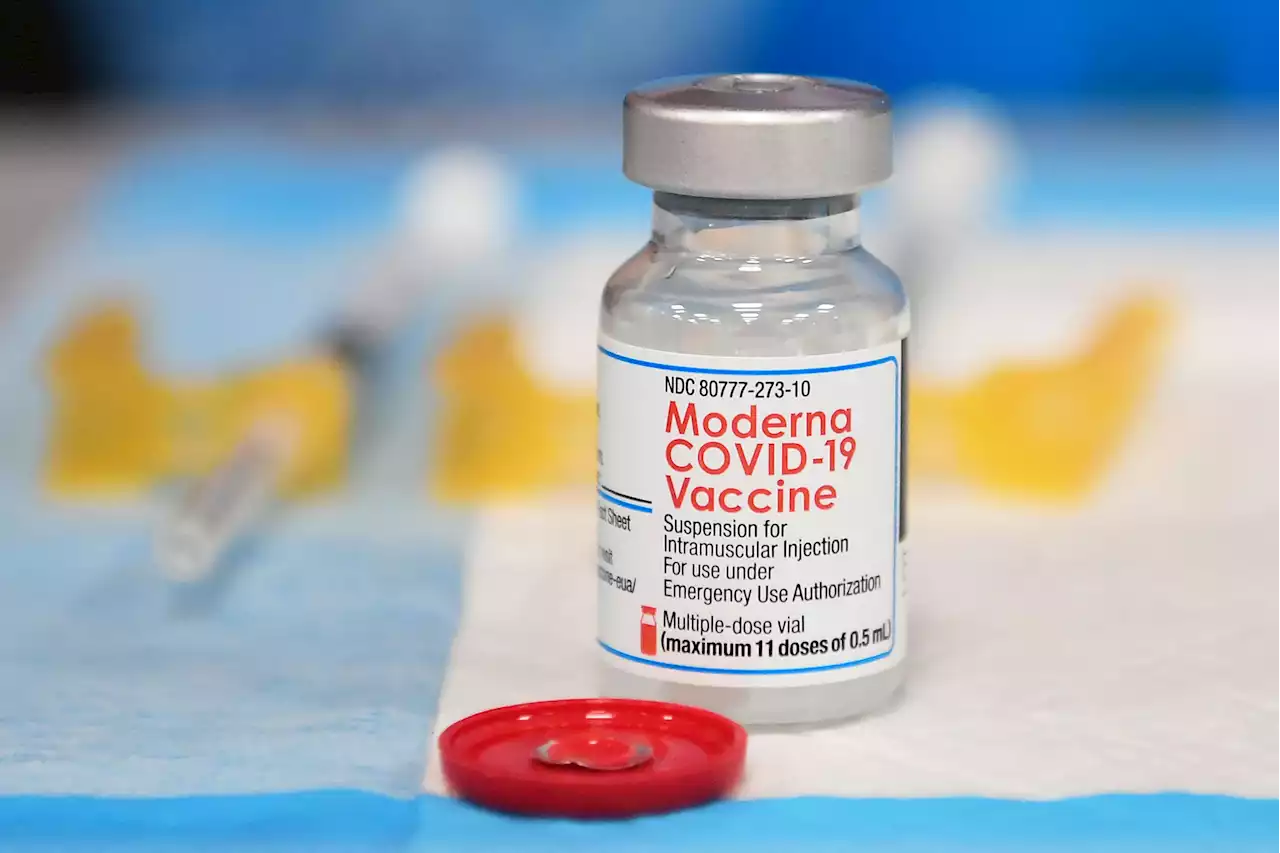 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
Read more »
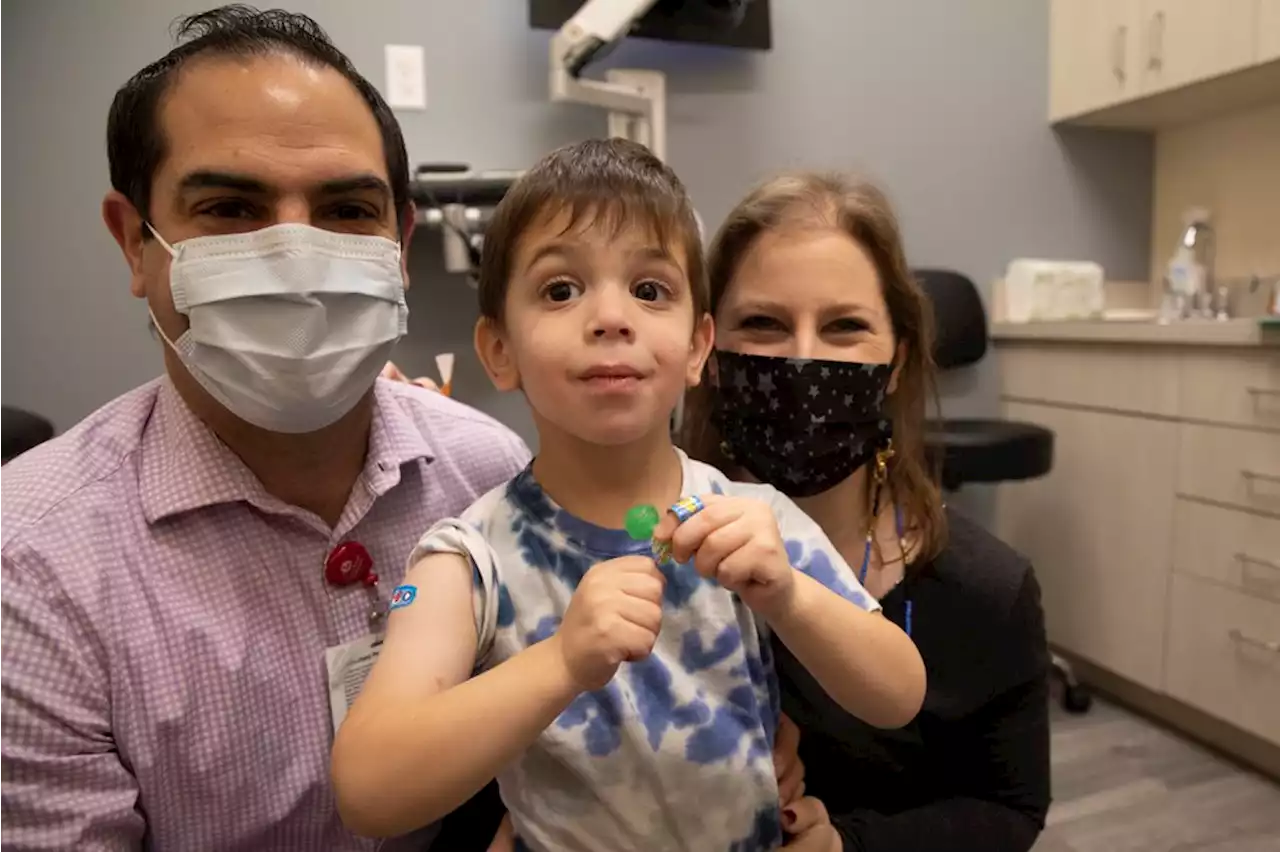 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Read more »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Read more »
 FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
Read more »