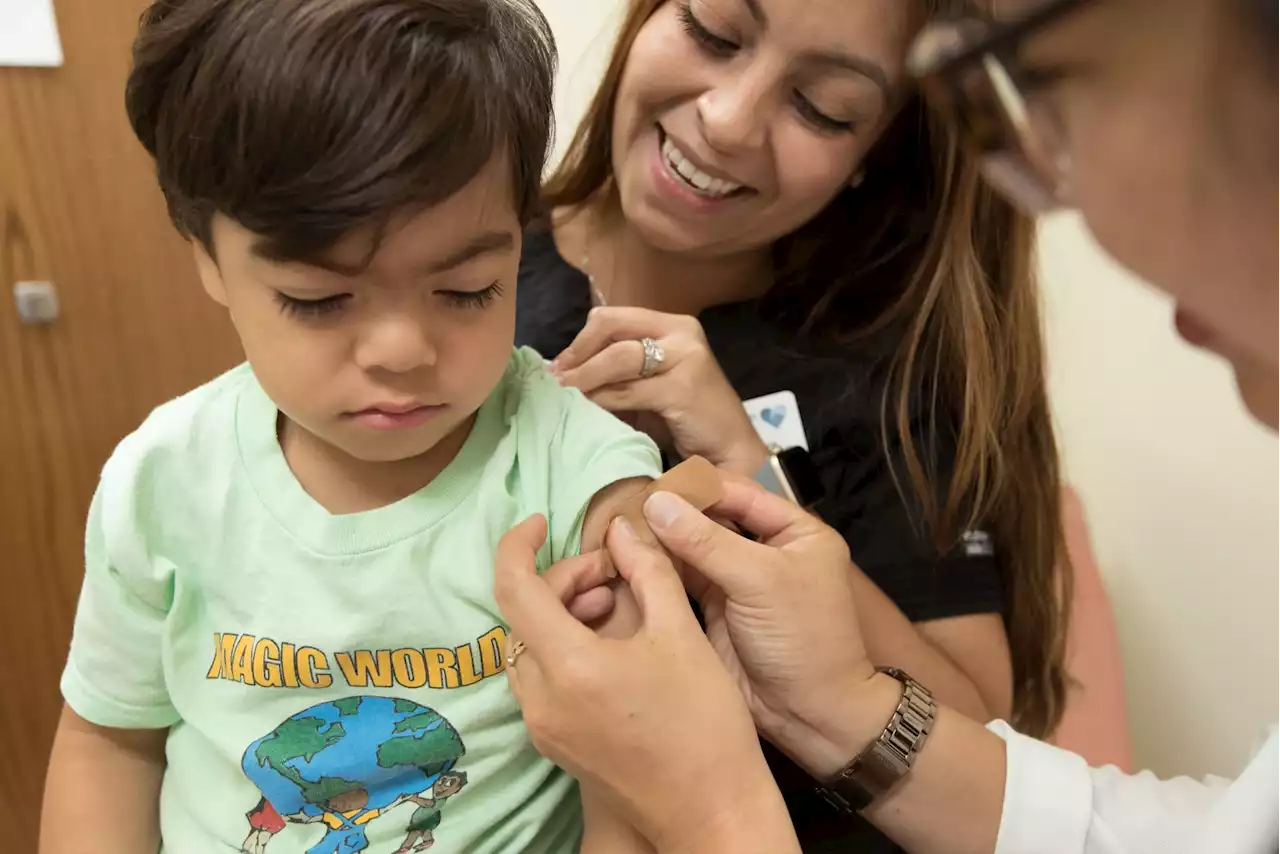
Following the endorsement, shots should become available to the general public within weeks.
Data presented at the June 16 panel clarified the February Pfizer decision. Two doses of the vaccine only reduced symptomatic illness in the younger population by 14 percent—a weak result, even ignoring the extremely high bar of 90-plus percent efficacy set by the adult mRNA vaccines. But with three doses, Pfizer has now increased the efficacy against symptomatic illness to 80 percent. Meanwhile, Moderna’s two-dose regimen showed a 40 percent reduction in kids’ chances of falling ill.
Both of the efficacy findings are bracketed by huge amounts of uncertainty. While the companies included tens of thousands of participants in clinical trials for adults’ vaccines, they only had several thousand for each of the kids’ vaccines. At the same time, the trials only tested for COVID in kids who exhibited symptoms, even thoughare asymptomatic.
But the FDA advisors were convinced of the vaccines’ efficacies, in part, because of the antibody levels in vaccinated children that participated in the trials. Those antibodies roughly corresponded to what’s seen in adults, and representatives for the companies said they believed the pediatric vaccines would confer similar real-world protection against severe illness.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Read more »
 FDA committee recommends COVID shots for kids as young as 6 monthsA key FDA advisory committee voted unanimously to recommend Moderna's COVID vaccine receive emergency use authorization for kids as young as 6 months old. They have another vote scheduled today for the Pfizer-BioNTech vaccine.
FDA committee recommends COVID shots for kids as young as 6 monthsA key FDA advisory committee voted unanimously to recommend Moderna's COVID vaccine receive emergency use authorization for kids as young as 6 months old. They have another vote scheduled today for the Pfizer-BioNTech vaccine.
Read more »
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Read more »
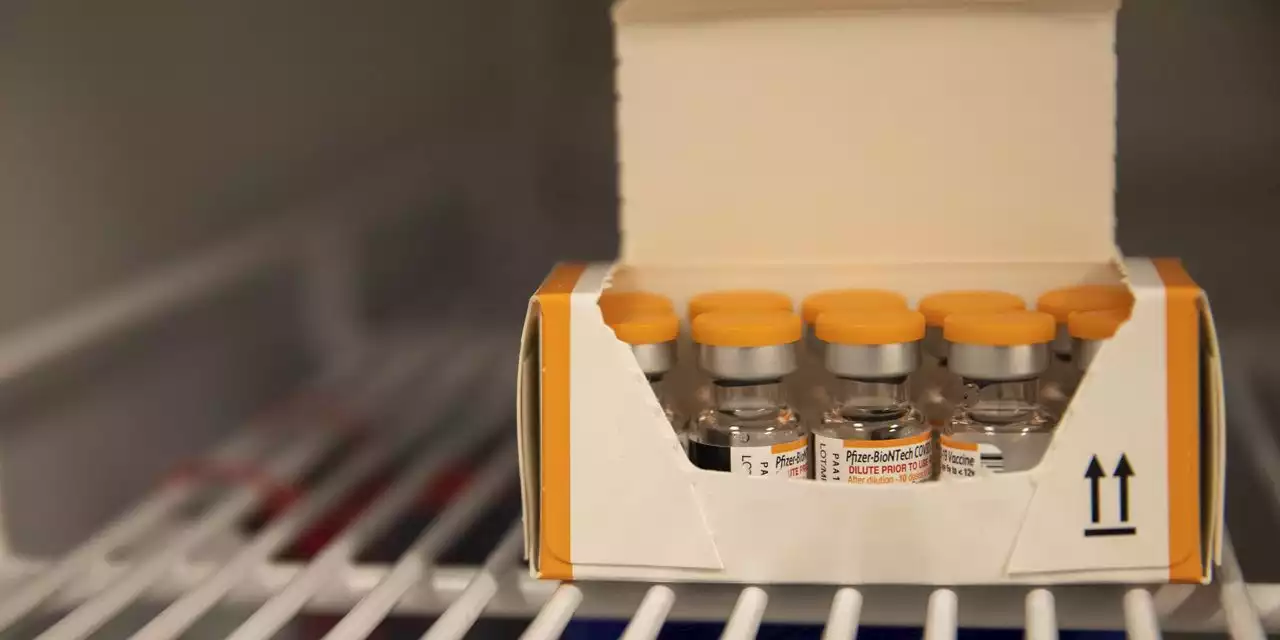 FDA Advisers Review Pfizer, Moderna Covid-19 Vaccines in Young ChildrenThe advisory panel’s meeting moves the Pfizer and Moderna shots one step closer to authorization for one of the last groups of people in the U.S. who haven’t been able to get vaccinated against the coronavirus.
FDA Advisers Review Pfizer, Moderna Covid-19 Vaccines in Young ChildrenThe advisory panel’s meeting moves the Pfizer and Moderna shots one step closer to authorization for one of the last groups of people in the U.S. who haven’t been able to get vaccinated against the coronavirus.
Read more »