The FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
The Food and Drug Administration ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine - a step not expected to require waiting on new studies. For now at least. BA.5 currently is causing nearly all COVID-19 infections in the U.S. and much of the world. There's no way to know if it still will be a threat this winter -- or if another mutant will have replaced it.
The news comes after Britain a week ago became the first in the world to authorize a different update to Moderna's COVID-19 vaccines -- shots that add protection against the original omicron that struck last winter. Pfizer previously announced results from a study that found its earlier omicron tweak significantly revved up antibodies capable of fighting the first omicron version, called BA.1, and to a lesser degree the genetically distinct BA.4 and BA.5 omicron relatives. Its application to the FDA contains that data plus animal testing of the newest recipe update.Pfizer and BioNTech expect to start a trial using the BA.4 and BA.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer-BioNTech Submits the First Omicron COVID-19 Booster for FDA AuthorizationPfizer-BioNTech Submit the First Omicron COVID-19 Booster for FDA Authorization
Pfizer-BioNTech Submits the First Omicron COVID-19 Booster for FDA AuthorizationPfizer-BioNTech Submit the First Omicron COVID-19 Booster for FDA Authorization
Read more »
 Pfizer, BioNTech Seek FDA Authorization for Updated Covid-19 VaccineThe updated shot, which targets the latest Omicron subvariants as well as the original virus, would be used in a fall booster campaign.
Pfizer, BioNTech Seek FDA Authorization for Updated Covid-19 VaccineThe updated shot, which targets the latest Omicron subvariants as well as the original virus, would be used in a fall booster campaign.
Read more »
 Pfizer Asks FDA To Greenlight New Omicron Booster Shots, Which Could Arrive This FallPfizer and BioNTech have asked the FDA to authorize an updated version of their COVID-19 vaccine for this fall — this one designed specifically to target the omicron subvariants that are dominant in the U.S.
Pfizer Asks FDA To Greenlight New Omicron Booster Shots, Which Could Arrive This FallPfizer and BioNTech have asked the FDA to authorize an updated version of their COVID-19 vaccine for this fall — this one designed specifically to target the omicron subvariants that are dominant in the U.S.
Read more »
 Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Read more »
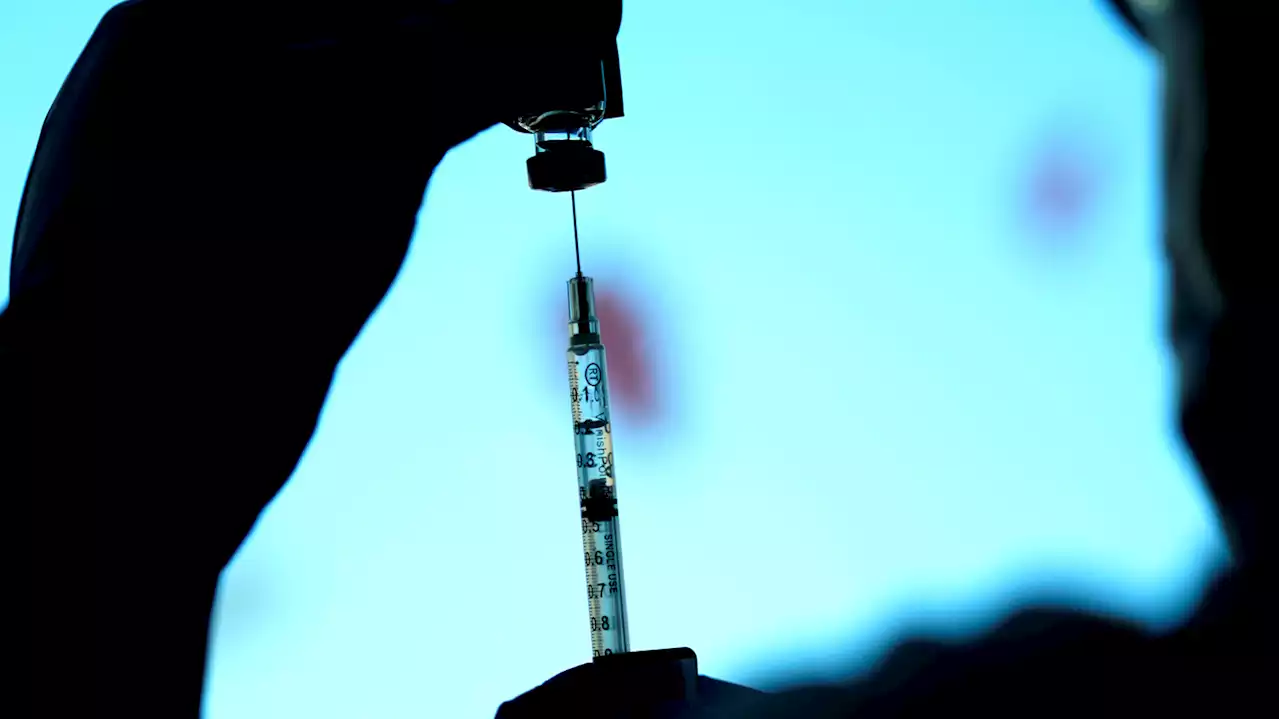 Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Read more »
 Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Read more »
