Pfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
The new booster shot would be an update to Pfizer's current version of the shot, which was designed for the original strain of the coronavirus. On Monday, the drugmakers Pfizer and BioNTech announced that they've asked the Food and Drug Administration to authorize an updated version of their COVID-19 vaccine — this one designed specifically to target the omicron subvariants that are dominant in the U.S.
If the vaccine is authorized by the FDA, distribution could start "immediately" to help the country prepare for potential fall and winter surges of the coronavirus, Pfizer CEO Albert Bourla said in a statement. Instead of waiting for results from human trials, the FDA asked the drug companies to initially submit only the results of tests on mice,Regulators will rely on those results — along with the human neutralizing antibody data from earlier BA.1 bivalent booster studies — to decide whether to authorize the boosters.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Myocarditis Risk Higher After COVID-19 Infection than After COVID-19 VaccinationThe risk of myocarditis is substantially higher in the 4 weeks after COVID-19 infection than after a first dose of a COVID-19 vaccine, say researchers of new study. CardioTwitter
Myocarditis Risk Higher After COVID-19 Infection than After COVID-19 VaccinationThe risk of myocarditis is substantially higher in the 4 weeks after COVID-19 infection than after a first dose of a COVID-19 vaccine, say researchers of new study. CardioTwitter
Read more »
 Pfizer Seeks OK Of Updated COVID Vaccine Booster For FallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Pfizer Seeks OK Of Updated COVID Vaccine Booster For FallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Read more »
 Pfizer-BioNTech Submits the First Omicron COVID-19 Booster for FDA AuthorizationPfizer-BioNTech Submit the First Omicron COVID-19 Booster for FDA Authorization
Pfizer-BioNTech Submits the First Omicron COVID-19 Booster for FDA AuthorizationPfizer-BioNTech Submit the First Omicron COVID-19 Booster for FDA Authorization
Read more »
 Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Read more »
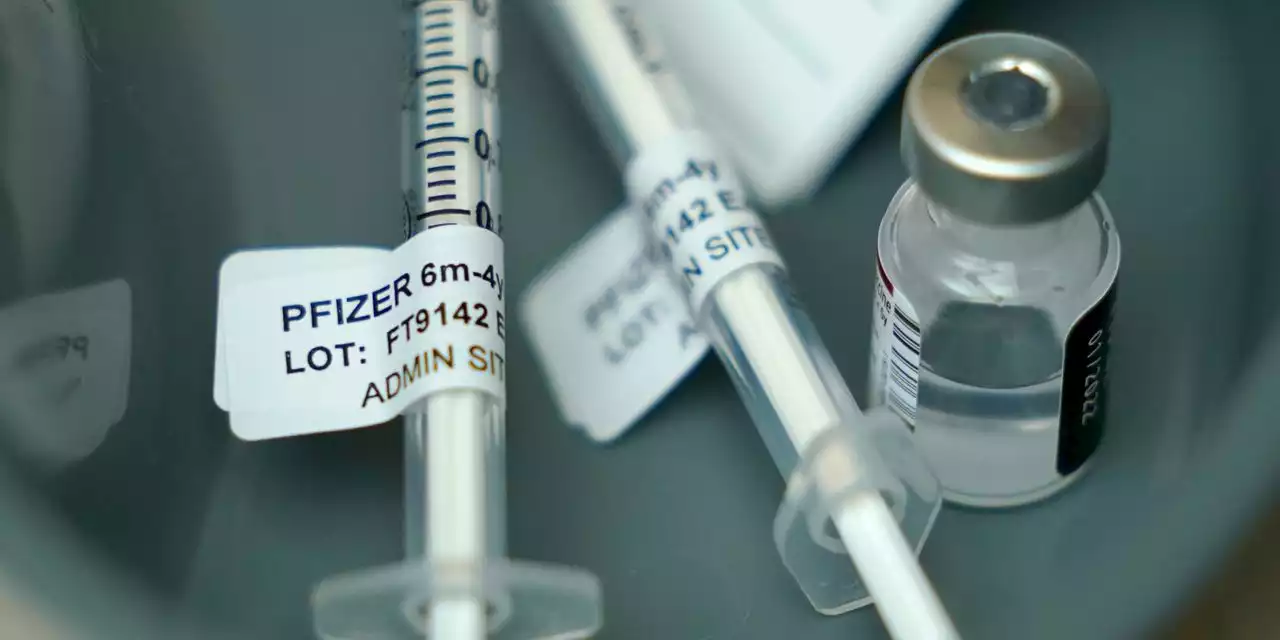 Pfizer, BioNTech Seek FDA Authorization for Updated Covid-19 VaccineThe updated shot, which targets the latest Omicron subvariants as well as the original virus, would be used in a fall booster campaign.
Pfizer, BioNTech Seek FDA Authorization for Updated Covid-19 VaccineThe updated shot, which targets the latest Omicron subvariants as well as the original virus, would be used in a fall booster campaign.
Read more »
 Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives
Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives
Read more »