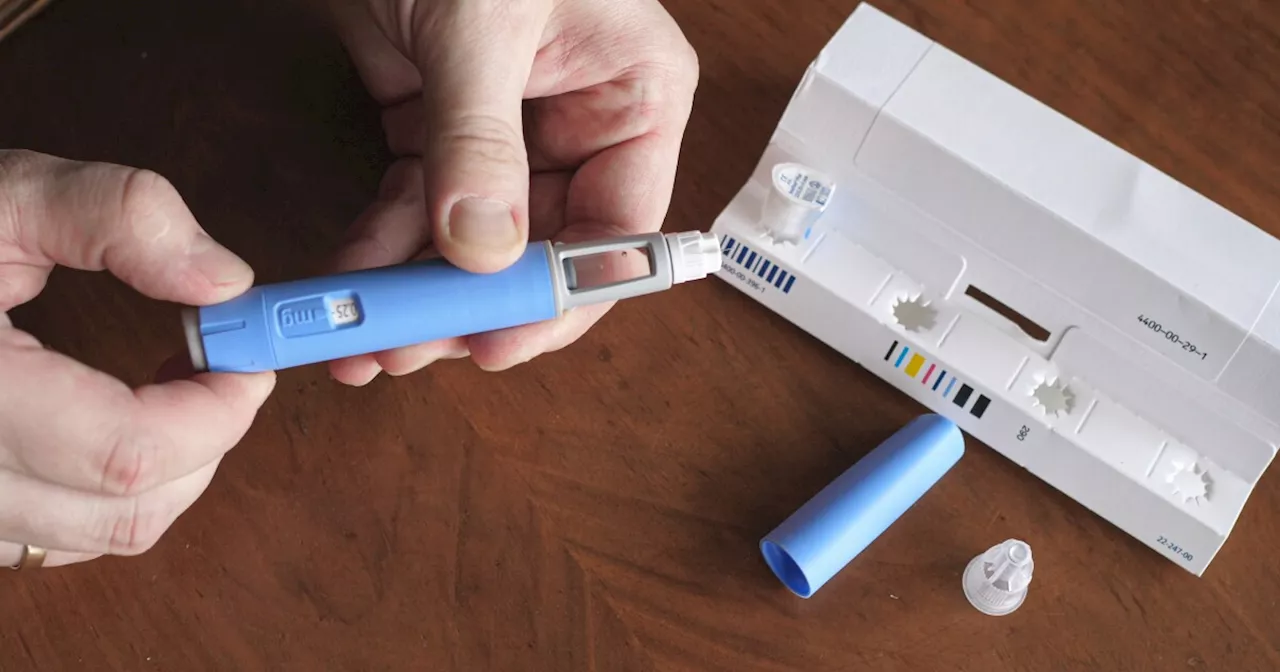
The Federal Drug Administration (FDA) has issued warnings to consumers regarding unapproved alternatives to semaglutide, a popular weight loss drug sold under brand names Ozempic and Wegovy. With shortages and high costs driving consumers to seek other options, experts caution against compounded versions which lack the same level of control.
DENVER — The Federal Drug Administration is warning consumers about unapproved semaglutide, a popular weight loss drug associated with the brand names Ozempic and Wegovy.Over the past few months, the FDA has received reports of weight loss drug shortages, prompting consumers to search for alternatives.“None of these drugs are old enough yet to be generic.
So compounded medicines are not FDA-approved. They don't have the same FDA regulation. It doesn't necessarily mean they're bad, but they're not FDA approved, so you don't have the same controls in place.”A compounded drug is a medicine pharmacies make from scratch.The FDA has received 364 reports of adverse events with compounded semaglutides and suggests that consumers only purchase the drug from state-approved pharmacies.
FDA WARNING Semaglutide WEIGHT LOSS DRUGS GENERIC ALTERNATIVES HEALTH RISKS
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA OKs New Drug for Urinary Tract InfectionsThe FDA has approved Orlynvah, a new oral treatment for uncomplicated urinary tract infections (UTIs) in women who have limited options for effective antibiotic therapy.
FDA OKs New Drug for Urinary Tract InfectionsThe FDA has approved Orlynvah, a new oral treatment for uncomplicated urinary tract infections (UTIs) in women who have limited options for effective antibiotic therapy.
Read more »
 US FDA Approves Journey Medical's Drug for Long-term Skin ConditionJourney Medical said on Monday the U.S. Food and Drug Administration has approved its drug for the treatment of a long-term skin condition called rosacea, sending the...
US FDA Approves Journey Medical's Drug for Long-term Skin ConditionJourney Medical said on Monday the U.S. Food and Drug Administration has approved its drug for the treatment of a long-term skin condition called rosacea, sending the...
Read more »
 Nationwide Supplement Recall After FDA Discovers 'Hidden Drug Ingredient'Adult males who use nitrate-based medication to manage heart conditions are the most at risk demographic, the agency said
Nationwide Supplement Recall After FDA Discovers 'Hidden Drug Ingredient'Adult males who use nitrate-based medication to manage heart conditions are the most at risk demographic, the agency said
Read more »
 New FDA rules for TV drug ads: Simpler language and no distractionsNew rules require drugmakers to be clearer about explaining the risks and side effects of prescription drugs.
New FDA rules for TV drug ads: Simpler language and no distractionsNew rules require drugmakers to be clearer about explaining the risks and side effects of prescription drugs.
Read more »
 New FDA rules for TV drug ads: Simpler language and no distractionsNew rules require drugmakers to be clearer about explaining the risks and side effects of prescription drugs. The new guidelines from the the Food and Drug Administration are designed to do away with pharmaceutical industry practices that downplay or distract viewers from risk information.
New FDA rules for TV drug ads: Simpler language and no distractionsNew rules require drugmakers to be clearer about explaining the risks and side effects of prescription drugs. The new guidelines from the the Food and Drug Administration are designed to do away with pharmaceutical industry practices that downplay or distract viewers from risk information.
Read more »
 New FDA rules for TV drug ads: Simpler language and no distractionsNew rules require drugmakers to be clearer about explaining the risks and side effects of prescription drugs.
New FDA rules for TV drug ads: Simpler language and no distractionsNew rules require drugmakers to be clearer about explaining the risks and side effects of prescription drugs.
Read more »