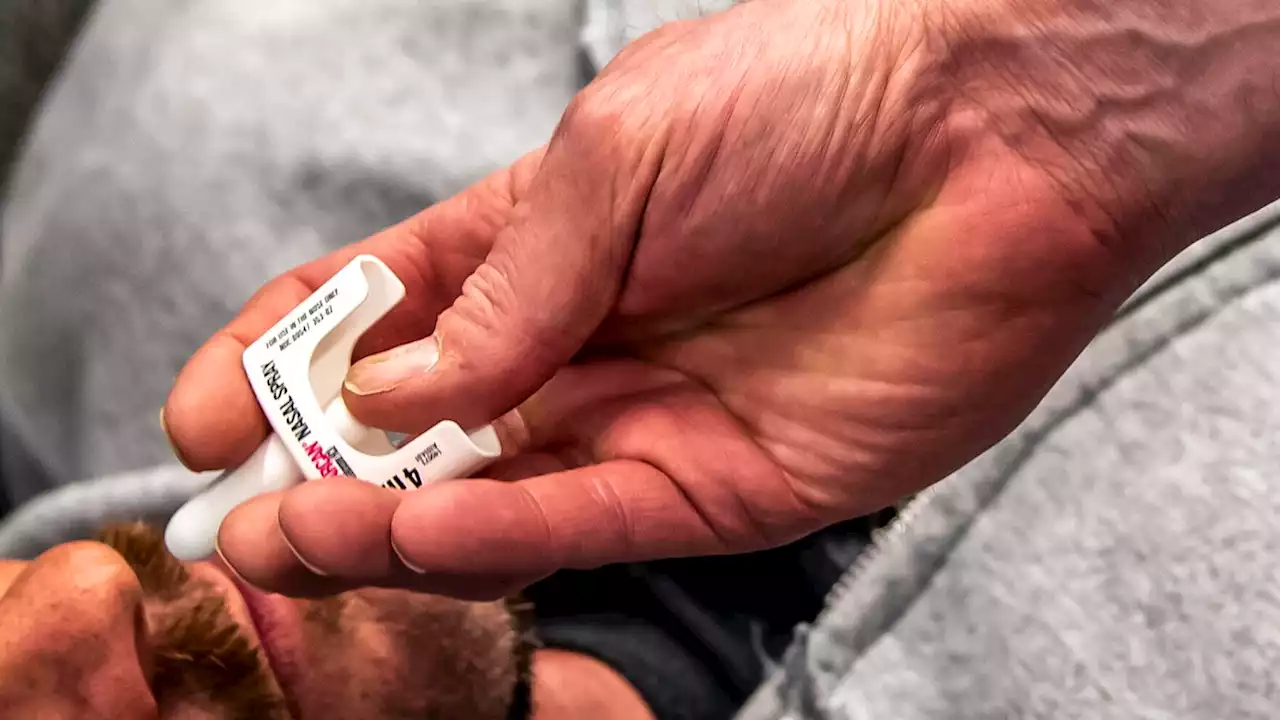
The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
WASHINGTON — The overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health advisers said Wednesday.
The positive vote, which is not binding, came despite concerns from some panel members about the drug’s instructions and packaging, which caused confusion among some people in a company study. People are also reading… Panel members urged the FDA to move swiftly rather than waiting for Emergent to conduct a follow-up study with the easier-to-understand label.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA considers making Narcan opioid overdose antidote available without prescription | CNNAdvisers to the US Food and Drug Administration will meet Wednesday to discuss whether a nasal spray version of the opioid overdose antidote Narcan should be made available over-the-counter.
FDA considers making Narcan opioid overdose antidote available without prescription | CNNAdvisers to the US Food and Drug Administration will meet Wednesday to discuss whether a nasal spray version of the opioid overdose antidote Narcan should be made available over-the-counter.
Read more »
 Narcan overdose antidote close to being sold over the counter: FDA meetingHealth experts say a higher accessibility to the drug could help combat the current opioid crisis in the US
Narcan overdose antidote close to being sold over the counter: FDA meetingHealth experts say a higher accessibility to the drug could help combat the current opioid crisis in the US
Read more »
 FDA Panel Backs Moving Opioid Antidote Narcan Over the CounterU.S. health advisers say the overdose-reversal drug naloxone should be made available as an over-the-counter medication. It’s the latest government proposal to increase use of the medication against the opioid overdose crisis. The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations. The positive vote by an Food and Drug Administration advisory panel came despite difficulties understanding how to use the drug in a company study. FDA will make a final decision on the drug in coming weeks.
FDA Panel Backs Moving Opioid Antidote Narcan Over the CounterU.S. health advisers say the overdose-reversal drug naloxone should be made available as an over-the-counter medication. It’s the latest government proposal to increase use of the medication against the opioid overdose crisis. The nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations. The positive vote by an Food and Drug Administration advisory panel came despite difficulties understanding how to use the drug in a company study. FDA will make a final decision on the drug in coming weeks.
Read more »
 FDA panel backs moving opioid antidote Narcan over the counterThe nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
FDA panel backs moving opioid antidote Narcan over the counterThe nasal spray version, Narcan, is already available without a prescription in all 50 states. But switching it to over-the-counter status would allow it to be sold in vending machines, supermarkets and other locations.
Read more »
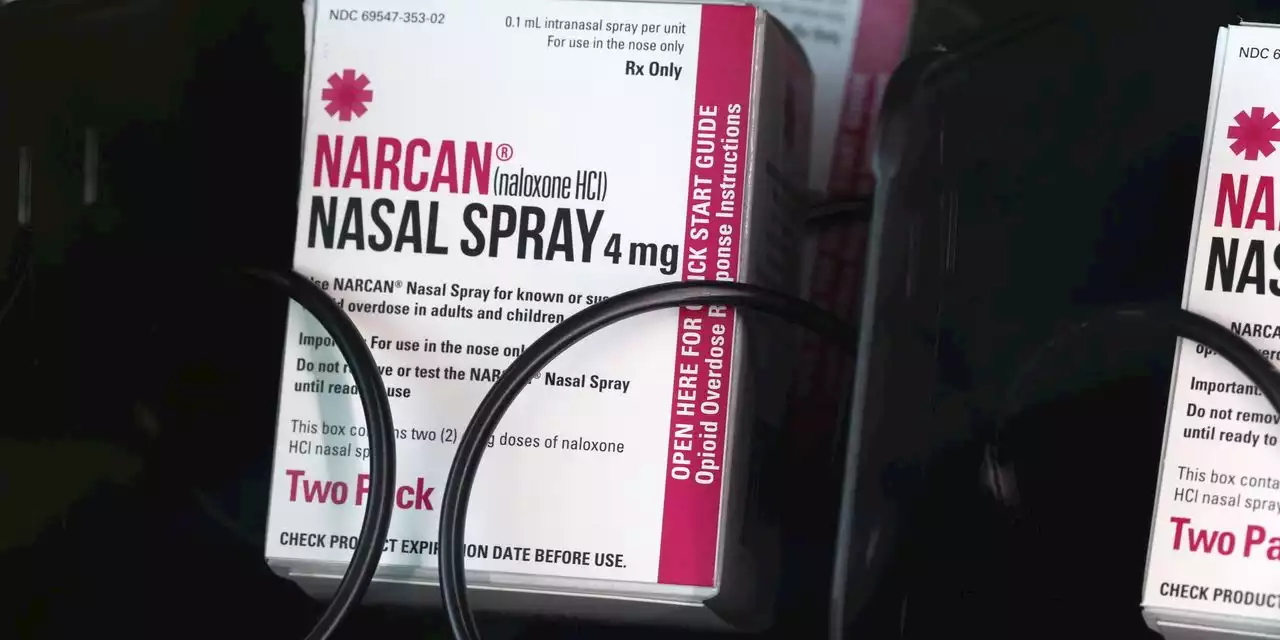 FDA panel says opioid antidote Narcan should be available over the counterThe overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health...
FDA panel says opioid antidote Narcan should be available over the counterThe overdose-reversal drug naloxone should be available as an over-the-counter medication to aid the national response to the opioid crisis, U.S. health...
Read more »
 1 in 10 new drugs don't achieve their main goals despite FDA approval, study findsThe study by Harvard and Yale researchers found 10% of FDA approved drugs were based on studies that missed their goals.
1 in 10 new drugs don't achieve their main goals despite FDA approval, study findsThe study by Harvard and Yale researchers found 10% of FDA approved drugs were based on studies that missed their goals.
Read more »