U.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication.
The potential move represents the latest government effort to increase use of a medication that has been a key tool in the battle against the U.S. overdose epidemic that kills more than 100,000 people annually. The decades-old drug can counteract the effects of an opioid overdose in minutes.
Emergent presented results from a 70-person study designed to show that people of various ages and backgrounds could quickly and correctly understand how to use the device in an emergency. About a third of people in the study had low reading ability, a group that FDA said should have been larger. Emergent said it plans to move all the directions to a single panel and add pictograms, per FDA's suggestion.
“It’s going to make a tremendous impact on how people view the medication,” said Sheila Vakharia, deputy director of research and academic engagement at the Drug Policy Alliance. “It will help to destigmatize it and make people know it’s safe and easy to use.” U.S. overdose death rates began steadily climbing in the 1990s, driven by painkillers. Waves of deaths followed, led by other opioids like heroin and — most recently — illicit fentanyl. Nearly 107,000 Americans died of drug overdoses in 2021, an all-time high, though recent data suggests deaths may be plateauing.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA considers making Narcan opioid overdose antidote available without prescription | CNNAdvisers to the US Food and Drug Administration will meet Wednesday to discuss whether a nasal spray version of the opioid overdose antidote Narcan should be made available over-the-counter.
FDA considers making Narcan opioid overdose antidote available without prescription | CNNAdvisers to the US Food and Drug Administration will meet Wednesday to discuss whether a nasal spray version of the opioid overdose antidote Narcan should be made available over-the-counter.
Read more »
 Narcan overdose antidote close to being sold over the counter: FDA meetingHealth experts say a higher accessibility to the drug could help combat the current opioid crisis in the US
Narcan overdose antidote close to being sold over the counter: FDA meetingHealth experts say a higher accessibility to the drug could help combat the current opioid crisis in the US
Read more »
 FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication
FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone should be made available as an over-the-counter medication
Read more »
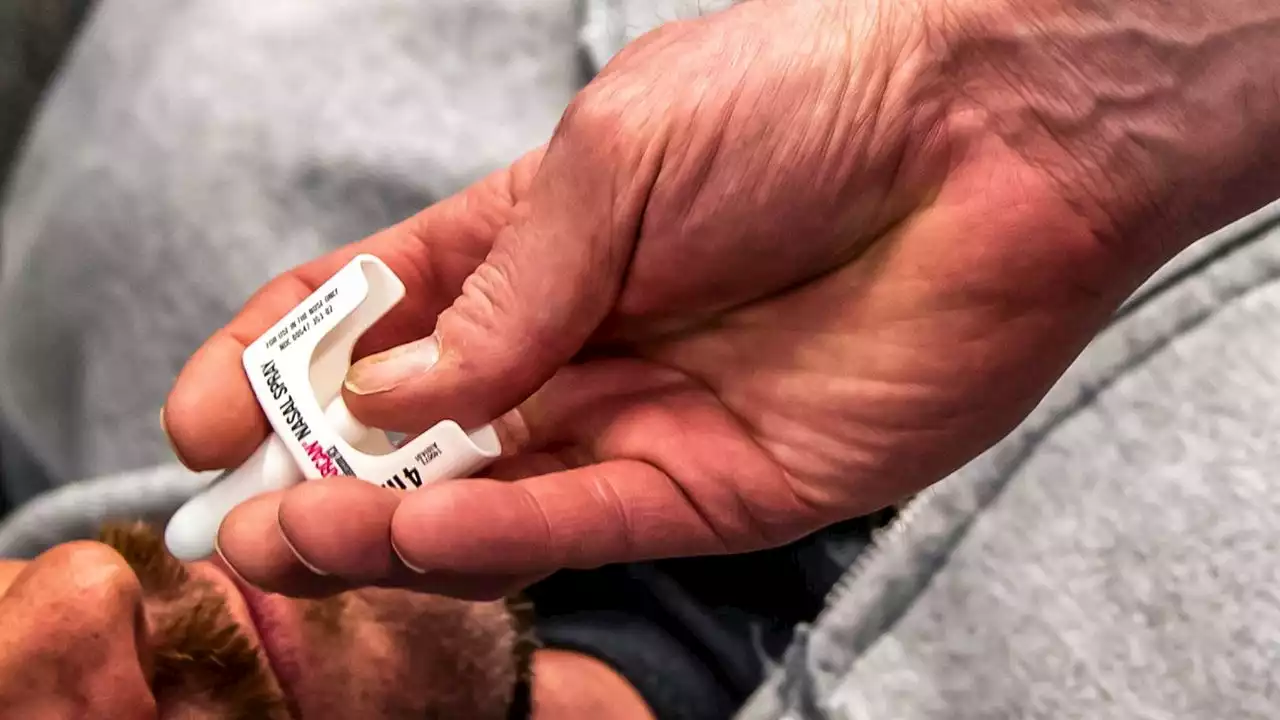 FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone, commonly referred to as Narcan, should be made available as an over-the-counter medication.
FDA panel weighs moving opioid antidote over the counterU.S. health advisers are weighing whether the overdose-reversal drug naloxone, commonly referred to as Narcan, should be made available as an over-the-counter medication.
Read more »
 1 in 10 new drugs don't achieve their main goals despite FDA approval, study findsThe study by Harvard and Yale researchers found 10% of FDA approved drugs were based on studies that missed their goals.
1 in 10 new drugs don't achieve their main goals despite FDA approval, study findsThe study by Harvard and Yale researchers found 10% of FDA approved drugs were based on studies that missed their goals.
Read more »
 1 in 10 new drugs don't achieve their main goals despite FDA approval, study findsOne in 10 new drugs were cleared by federal drug regulators in recent years based on studies that didn't achieve their main goals, a new study shows.
1 in 10 new drugs don't achieve their main goals despite FDA approval, study findsOne in 10 new drugs were cleared by federal drug regulators in recent years based on studies that didn't achieve their main goals, a new study shows.
Read more »
