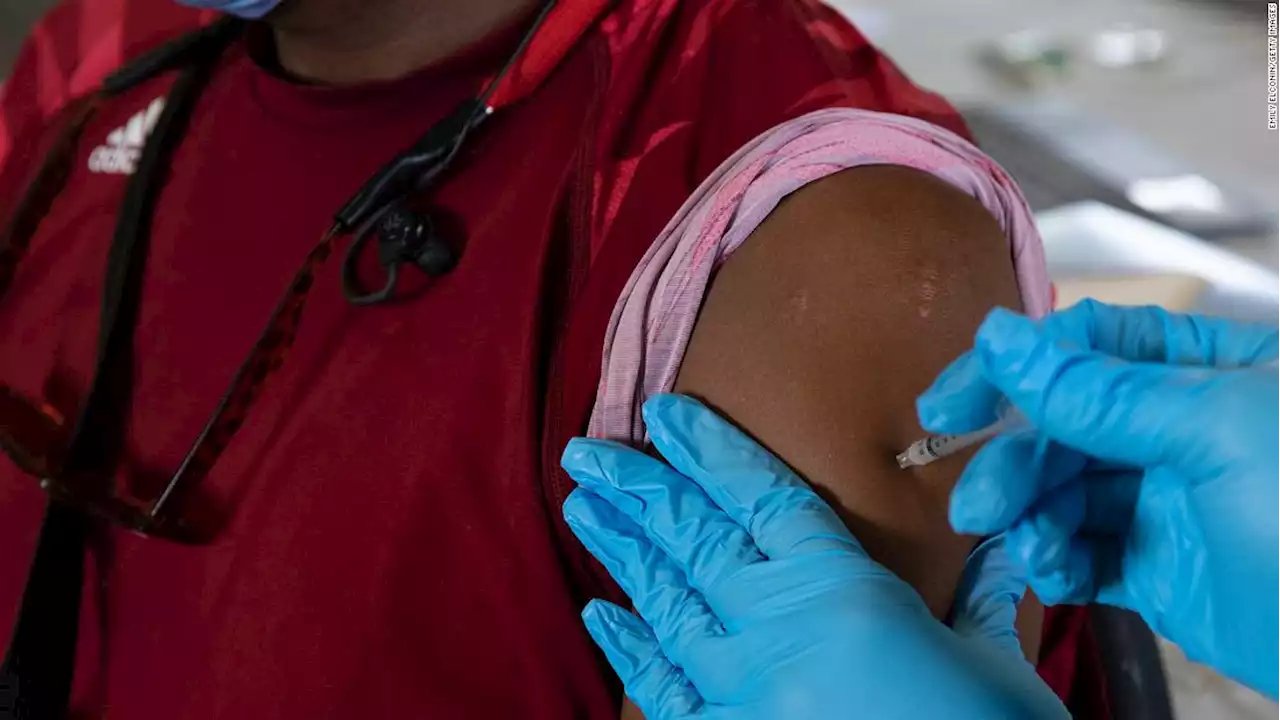
The FDA has expanded the emergency use authorization of the Pfizer and Moderna Covid-19 vaccines to allow adults age 50 and older to get a second booster as early as four months after their first booster dose of any Covid-19 vaccine. (via CNN)
The move extends the availability of additional boosters to healthy older adults. The FDA had previously allowed second booster shots for anyone 12 years of age or older who was severely immune deficient, starting four months after their first booster."Current evidence suggests some waning of protection over time against serious outcomes from COVID-19 in older and immunocompromised individuals.
A smaller study of health care workers, which included younger adults, found that fourth boosters were safe and restored antibodies to the same levels reached after third doses. But fourth doses were only moderately effective -- about 30 to 40% -- at preventing illness. And most of the workers who got sick still had high viral loads, suggested that they were capable of transmitting the infection to others.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
FDA OKs second Pfizer, Moderna COVID-19 booster for ages 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Read more »
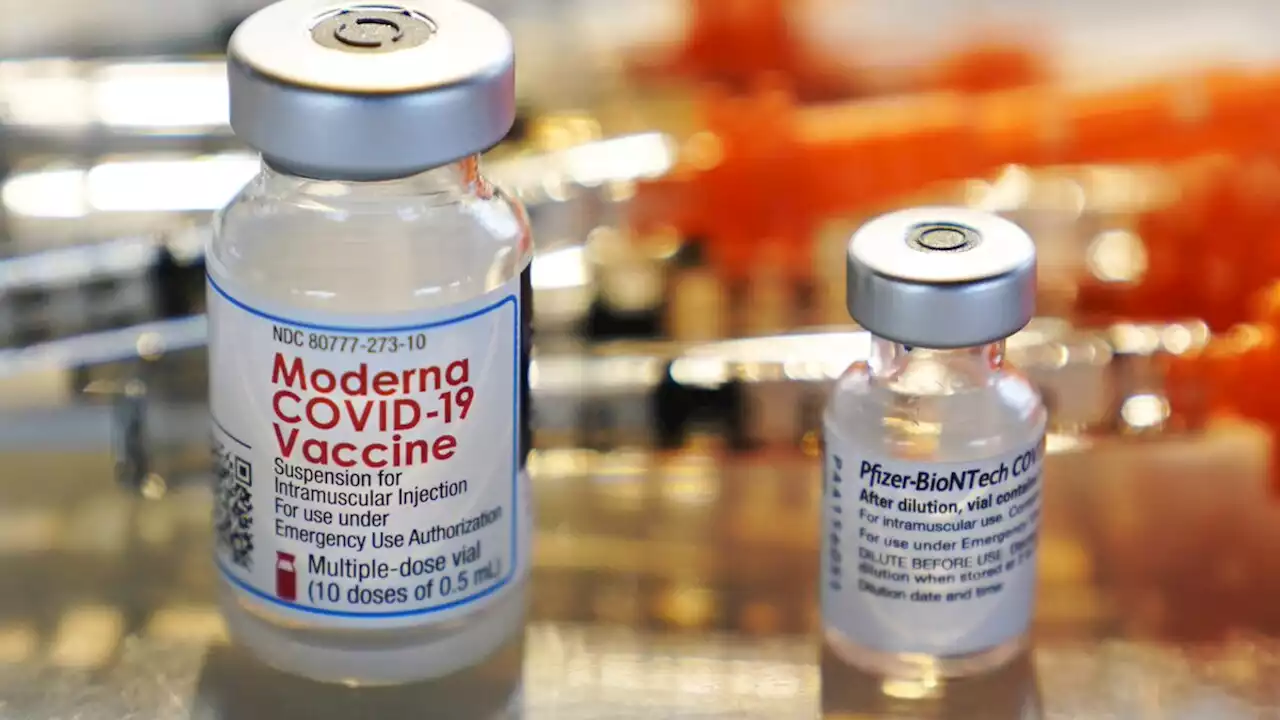 FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
Read more »
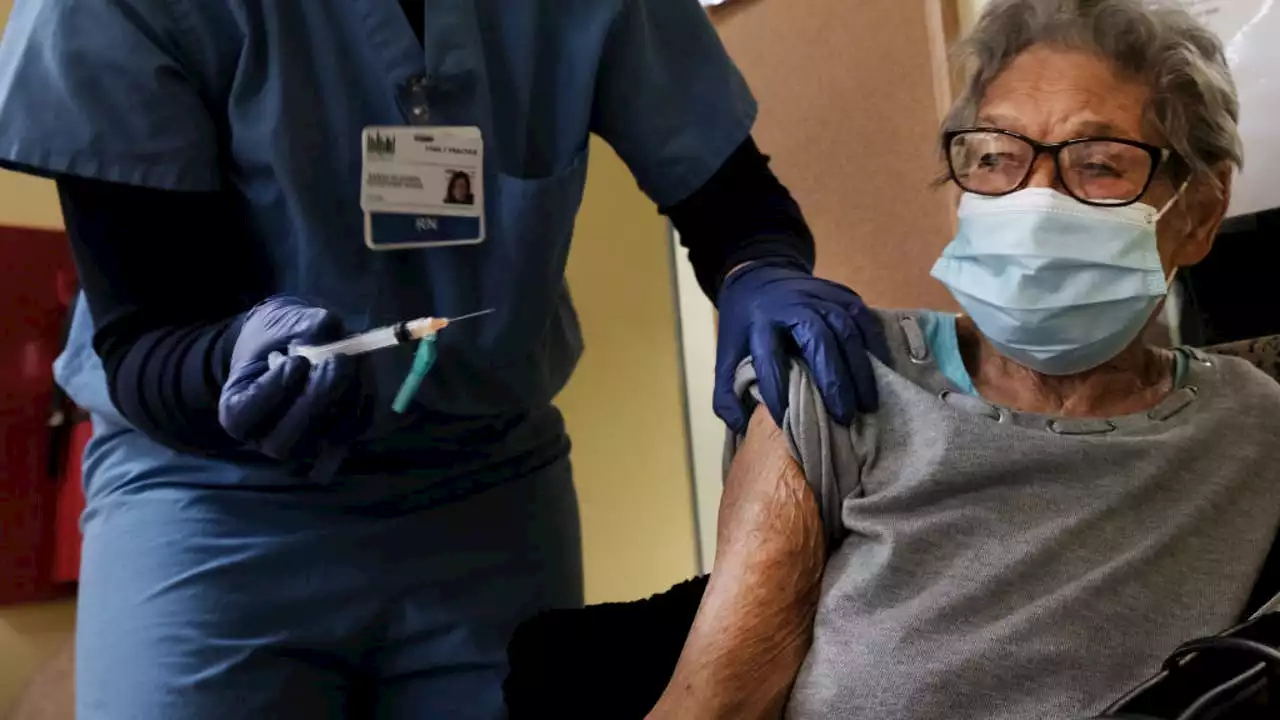 FDA approves 2nd COVID-19 vaccine booster for those 50 and olderU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
FDA approves 2nd COVID-19 vaccine booster for those 50 and olderU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
Read more »
 Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Read more »