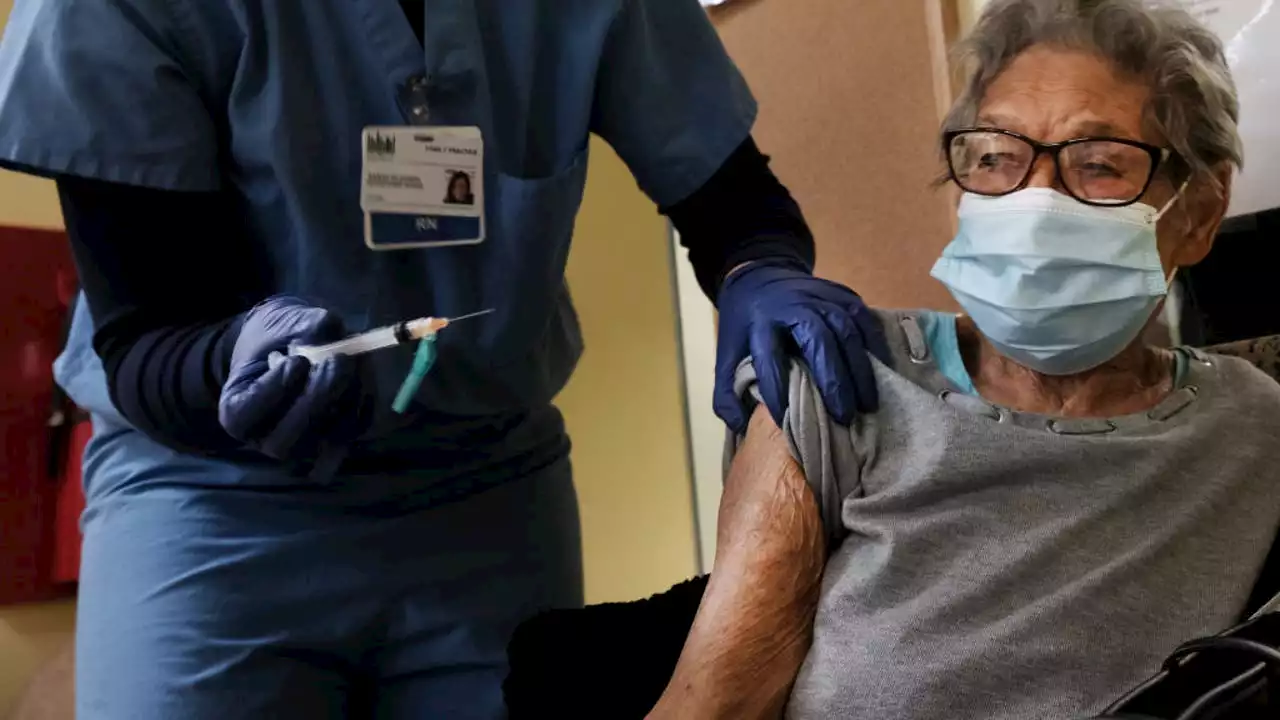
BREAKING: FDA approves 2nd COVID-19 vaccine booster for those 50 and older FOX13
Moderna asks FDA to add 4th COVID-19 vaccine booster dose for all adults to EUA
While authorities say the vaccinations continue to offer strong protection against severe illness, they haven’t held up as well against milder infections especially those due to the omicron mutant.
The U.S. booster campaign was based on evidence that the shots’ effectiveness, particularly against milder infections, was waning about six months after the last dose. Calls for a third shot grew once it became clear the vaccines weren’t as strong against mutants as they were against earlier versions of the virus.While some early data left unclear just how much benefit another shot offered — or for how long — Pfizer said that an analysis of health records of more than 1.
An expert group convened by the World Health Organization also said it "strongly supports urgent and broad access" to booster doses of COVID-19 vaccine amid the global spread of omicron. WHO said it is continuing to monitor the global spread of omicron, including a "stealth" version known as BA.2, which has been documented to have re-infected some people after an initial case of omicron. There’s mixed research on whether it causes more severe disease, but vaccines appear just as effective against it.Cathy Stoddard and the Associated Press contributed to this report. This story was reported from Los Angeles.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
FDA OKs second Pfizer, Moderna COVID-19 booster for ages 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Read more »
 FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
Read more »
 Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Covid-19: Two Covid-related deaths and 1,172 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,301.
Read more »
 Covid-19: Five Covid-related deaths and 1,204 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,306.
Covid-19: Five Covid-related deaths and 1,204 casesThe total number of deaths linked to Covid-19 in Northern Ireland since the start of the pandemic is 3,306.
Read more »