Arianna Johnson is a reporter on the Forbes news desk who covers explainers and trends, with a frequent focus on health and science. She joined Forbes in 2022 and works in Texas. Johnson has covered prominent weight loss drugs like Ozempic and Wegovy, the health effects of the artificial sweetener aspartame and the impacts of the Covid vaccines.
The Food and Drug Administration has approved updated COVID-19 vaccines to protect against currently circulating variants, and the shots have shown to be more effective than the now-available vaccines.new COVID-19 vaccines from Pfizer and Moderna on Thursday ahead of the 2024-2025 fall and winter respiratory virus season, and Novavax said it’s still working with the FDA to gain approval before peak vaccination season.
However, Biden administration officials are looking into acquiring permanent funding so a similar vaccine program will be offered to adults who don’t have insurance, the CDCThough the now-available vaccines—originally created to combat the XBB.1.5 variant—are effective at protecting against the JN lineage, the updated vaccines from all three manufacturers offered greater protection. Moderna’s KP.2 vaccine was up tomore effective at protecting mice against JN variants than its XBB.1.5 vaccine.
New Covid Vaccines Covid Boosters Covid Kp.2 Kp.3 Jn.1 When Will New Covid Vaccines Be Available New Covid Vaccine Effectiveness Covid Vaccine Age Limit
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
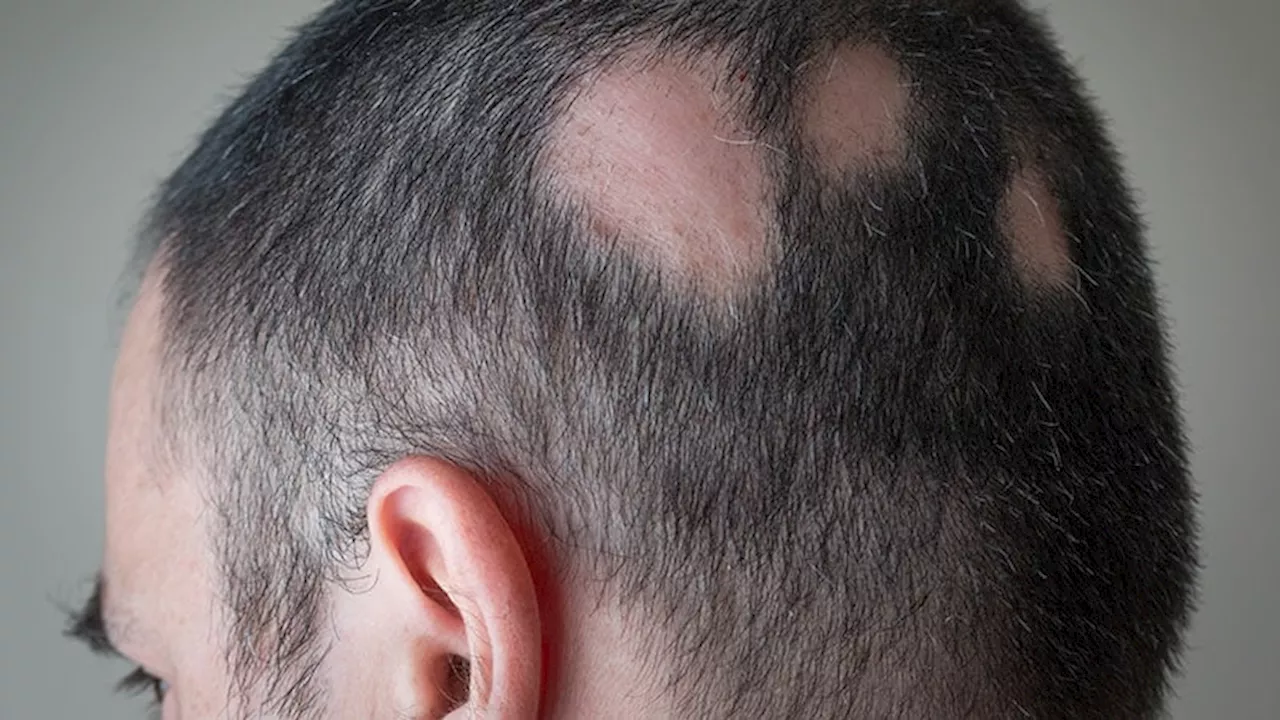 FDA Approves JAK Inhibitor Deuruxolitinib for Alopecia AreataThe pivotal phase 3 trials enrolled 1223 adults in the United States, Canada, and Europe.
FDA Approves JAK Inhibitor Deuruxolitinib for Alopecia AreataThe pivotal phase 3 trials enrolled 1223 adults in the United States, Canada, and Europe.
Read more »
 FDA approves new blood test to screen for colon cancerThe test showed an accuracy rate of 83 percent in clinical trials and could be a game changer in colon cancer screening, encouraging many more people to get checked for the disease. NBC News' Anne Thompson reports.
FDA approves new blood test to screen for colon cancerThe test showed an accuracy rate of 83 percent in clinical trials and could be a game changer in colon cancer screening, encouraging many more people to get checked for the disease. NBC News' Anne Thompson reports.
Read more »
 FDA approves blood test for colon cancerShield is approved as a screening method for those 45 and older who are at average risk for colon cancer, the second-leading cause of cancer-related death in the U.S.
FDA approves blood test for colon cancerShield is approved as a screening method for those 45 and older who are at average risk for colon cancer, the second-leading cause of cancer-related death in the U.S.
Read more »
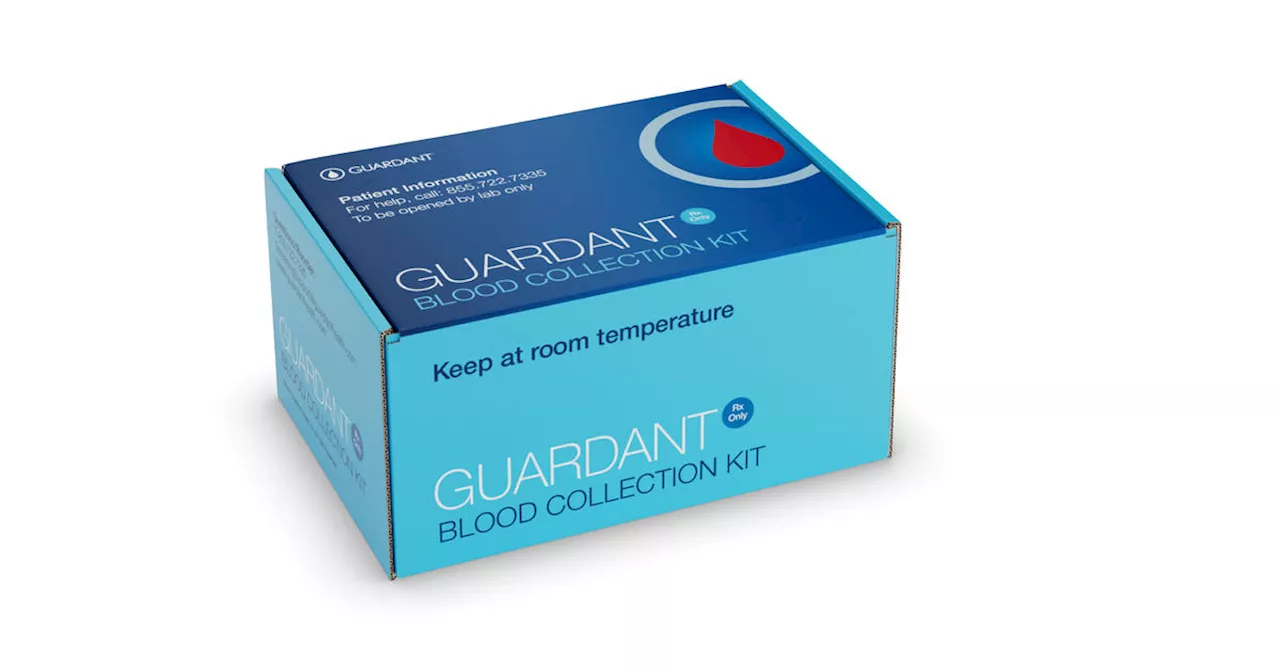 FDA approves blood test for colon cancer detectionThe Food and Drug Administration has approved a blood test intended to detect colorectal cancer, expanding options for screening for the potentially deadly disease.
FDA approves blood test for colon cancer detectionThe Food and Drug Administration has approved a blood test intended to detect colorectal cancer, expanding options for screening for the potentially deadly disease.
Read more »
 FDA approves colon cancer blood test, but some will still need colonoscopiesJustin Boggs is a writer for the E.W. Scripps company. Justin covers anything from politics to sports and entertainment.
FDA approves colon cancer blood test, but some will still need colonoscopiesJustin Boggs is a writer for the E.W. Scripps company. Justin covers anything from politics to sports and entertainment.
Read more »
 FDA approves blood test to detect colon cancer for those at 'average risk'The test detects cancer by detecting DNA shed by tumors in blood samples.
FDA approves blood test to detect colon cancer for those at 'average risk'The test detects cancer by detecting DNA shed by tumors in blood samples.
Read more »