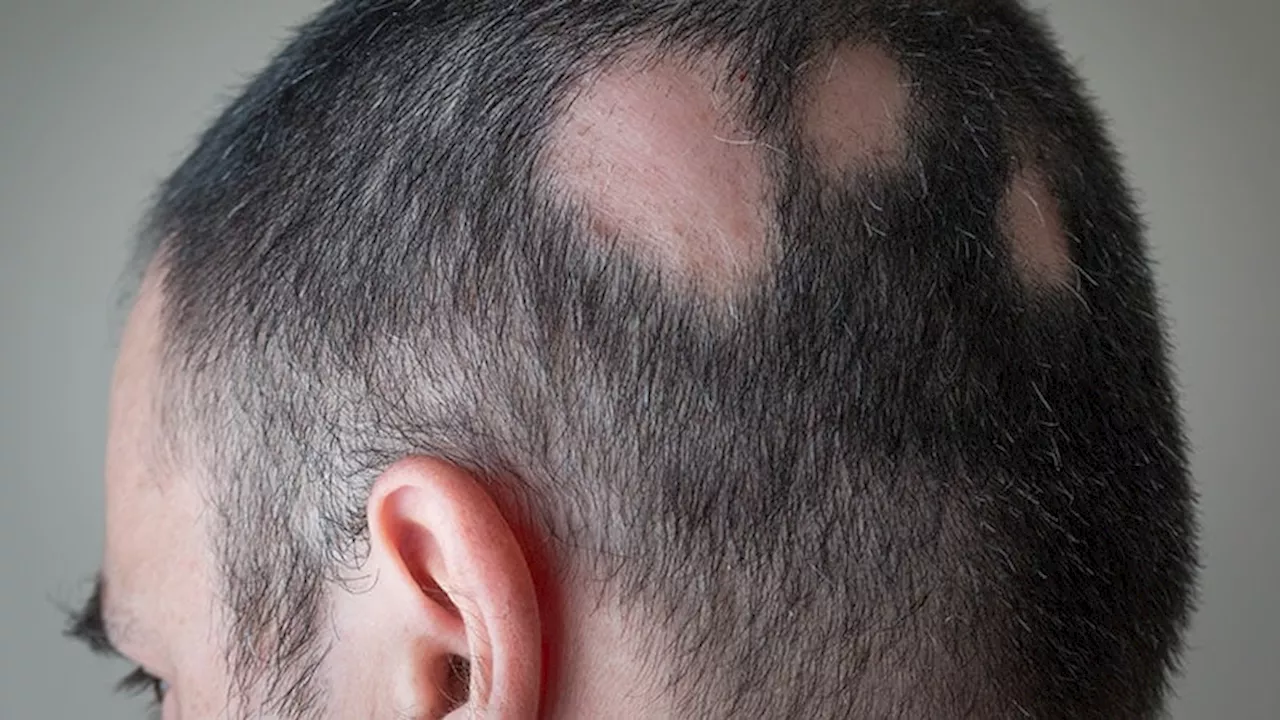
The pivotal phase 3 trials enrolled 1223 adults in the United States, Canada, and Europe.
The US Food and Drug Administration has approved the oral Janus kinase inhibitor deuruxolitinib for the treatment of adults with severefrom the drug's manufacturer Sun Pharma, is based on data from two pivotal randomized, double-blind, placebo-controlled phase 3 clinical trials :, which included 1223 adults with severe alopecia areata enrolled at sites in the United States, Canada, and Europe.
Deuruxolitinib, which comes in 8-mg tablets, is an oral selective inhibitor of JAK1 and JAK2 and is administered twice a day. According to the company press release, the average patient enrolled in the clinical trials had only 13% of their scalp hair coverage at baseline. At week 24, more than 30% of patients taking deuruxolitinib experiencing 80% or more scalp hair coverage . Also, up to 25% of patients had almost all of their scalp hair back at 24 weeks .
In terms of safety, the data showed that 3.1% of patients who received deuruxolitinib 8 mg twice daily in the phase 2 dose-ranging study and phase 3 randomized placebo-controlled trials discontinued treatment owing to adverse reactions. The three most common adverse events in placebo-controlled trials were, and nasopharyngitis . More than 100 people continued taking deuruxolitinib for more than 3 years.
Toxicology Toxicity Poisoning Toxins Alopecia Areata Hair Scalp Skin And Soft Tissue Infection Clinical Research Clinical Trials Clinical Studies Pre-Clinical Trial Double-Blind Study Double-Blind Studies Single-Blind Study Single-Blind Studies Healthcare And Medical Technology Health And Medical Tech Health And Med Tech Health And Medical Technology Healthcare Technology
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Tampons are the latest victim of shrinkflation, women allege — as makers say no way: 'Insidious'FDA approves new 'spiral' tampon shape
Tampons are the latest victim of shrinkflation, women allege — as makers say no way: 'Insidious'FDA approves new 'spiral' tampon shape
Read more »
 FDA Approves Epcoritamab for R/R Follicular LymphomaThe new approval follows the BiTE's first approval in 2023 for DLBCL.
FDA Approves Epcoritamab for R/R Follicular LymphomaThe new approval follows the BiTE's first approval in 2023 for DLBCL.
Read more »
 FDA Approves Third Ustekinumab BiosimilarThe biosimilar is approved for all indications of the reference medication, Stelara, and will launch in February 2025.
FDA Approves Third Ustekinumab BiosimilarThe biosimilar is approved for all indications of the reference medication, Stelara, and will launch in February 2025.
Read more »
 FDA approves new Alzheimer's treatment, donanemab from Eli LillyFDA approval of the new Alzheimer's treatment, which will be branded as Kisunla, follows years of setbacks.
FDA approves new Alzheimer's treatment, donanemab from Eli LillyFDA approval of the new Alzheimer's treatment, which will be branded as Kisunla, follows years of setbacks.
Read more »
 FDA approves Eli Lilly's Alzheimer’s drug that slows memory declineBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA approves Eli Lilly's Alzheimer’s drug that slows memory declineBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Read more »
 FDA approves Alzheimer’s drug donanemab after months of delayThe agency determined that the potential of slowing the devastating disease in patients with mild cognitive impairment outweigh the risks of brain bleeds.
FDA approves Alzheimer’s drug donanemab after months of delayThe agency determined that the potential of slowing the devastating disease in patients with mild cognitive impairment outweigh the risks of brain bleeds.
Read more »