The agent, which has returned to the US market in a reformulated version, is indicated for adults with relapsed or refractory stage 1-3 disease.
The US Food and Drug Administration has approved denileukin diftitox-cxdl for adults with relapsed or refractory stage 1-3 cutaneous T-cell lymphoma after at least one prior systemic therapy.), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. CitiusThis is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 through day 5 of 21-day cycles until disease progression or unacceptable toxicity.
Cutaneous T-Cell Lymphoma (CTCL) Non-Hodgkin's Lymphoma Non-Hodgkin Lymphoma NHL Skin Cancer Malignant Skin Neoplasm Lymphoma Malignant Lymphoma Toxicology Toxicity Poisoning Toxins Refractory Biologic Therapy Biologics Visual Impairment Vision Impairment Capillaries Impairment Pruritus Pruritis Itch Vision Care And Maintenance Interleukin-2 Il-2
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves Cambridge company's postpartum depression pillThe Food and Drug Administration on Friday granted approval of the drug, Zurzuvae, for adults experiencing severe depression related to childbirth or pregnancy. The pill is taken once a day for 14 days.
FDA approves Cambridge company's postpartum depression pillThe Food and Drug Administration on Friday granted approval of the drug, Zurzuvae, for adults experiencing severe depression related to childbirth or pregnancy. The pill is taken once a day for 14 days.
Read more »
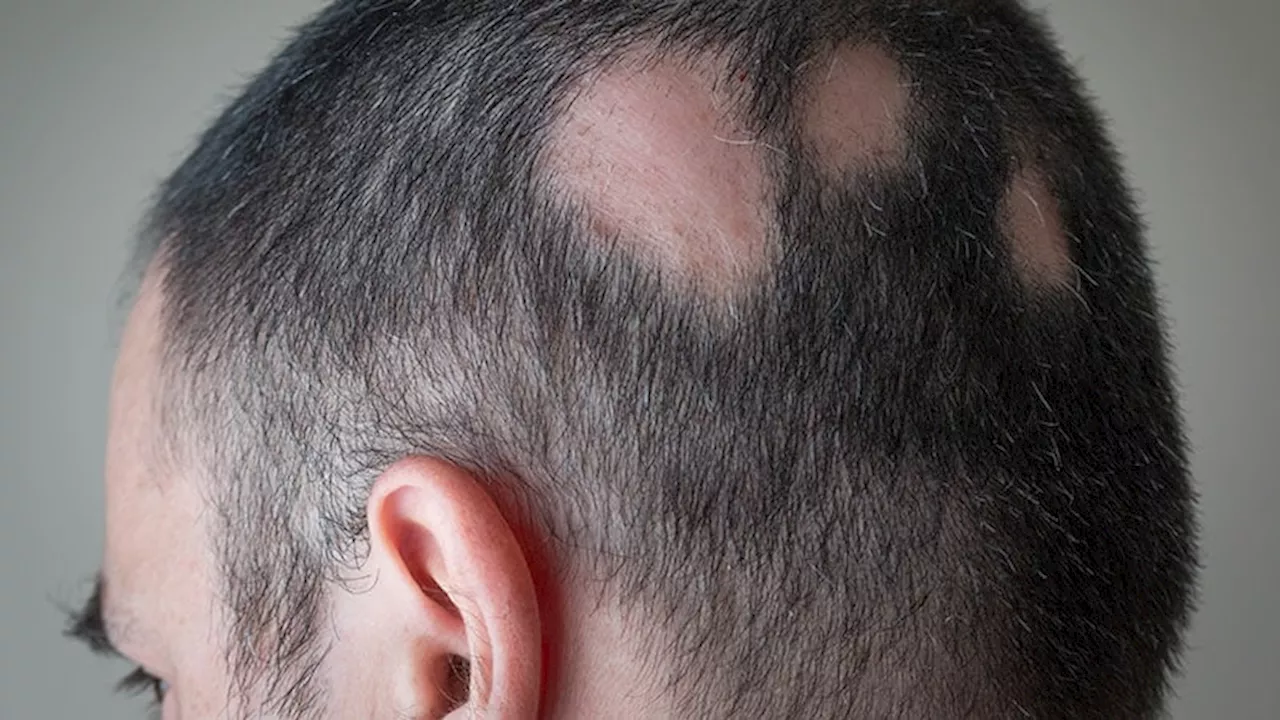 FDA Approves JAK Inhibitor Deuruxolitinib for Alopecia AreataThe pivotal phase 3 trials enrolled 1223 adults in the United States, Canada, and Europe.
FDA Approves JAK Inhibitor Deuruxolitinib for Alopecia AreataThe pivotal phase 3 trials enrolled 1223 adults in the United States, Canada, and Europe.
Read more »
 FDA approves new blood test to screen for colon cancerThe test showed an accuracy rate of 83 percent in clinical trials and could be a game changer in colon cancer screening, encouraging many more people to get checked for the disease. NBC News' Anne Thompson reports.
FDA approves new blood test to screen for colon cancerThe test showed an accuracy rate of 83 percent in clinical trials and could be a game changer in colon cancer screening, encouraging many more people to get checked for the disease. NBC News' Anne Thompson reports.
Read more »
 FDA approves blood test for colon cancerShield is approved as a screening method for those 45 and older who are at average risk for colon cancer, the second-leading cause of cancer-related death in the U.S.
FDA approves blood test for colon cancerShield is approved as a screening method for those 45 and older who are at average risk for colon cancer, the second-leading cause of cancer-related death in the U.S.
Read more »
 FDA approves blood test for colon cancer detectionThe Food and Drug Administration has approved a blood test intended to detect colorectal cancer, expanding options for screening for the potentially deadly disease.
FDA approves blood test for colon cancer detectionThe Food and Drug Administration has approved a blood test intended to detect colorectal cancer, expanding options for screening for the potentially deadly disease.
Read more »
 FDA approves colon cancer blood test, but some will still need colonoscopiesJustin Boggs is a writer for the E.W. Scripps company. Justin covers anything from politics to sports and entertainment.
FDA approves colon cancer blood test, but some will still need colonoscopiesJustin Boggs is a writer for the E.W. Scripps company. Justin covers anything from politics to sports and entertainment.
Read more »
