The nationwide recall of more than 7000 bottles of duloxetine delayed-release capsules is due to unacceptable levels of a potential carcinogen.
The recall is due to the detection of the nitrosamine impurity, N-nitroso duloxetine, above the proposed interim limit.
“If drugs contain levels of nitrosamines above the acceptable daily intake limits, FDA recommends these drugs be recalled by the manufacturer as appropriate,” the agency noted on its website. The affected bottles are from lot number 220128 with an expiration date of 12/2024 and NDC of 51991-746-05.“Healthcare professionals can educate patients about alternative treatment options to medications with potential nitrosamine impurities if available and clinically appropriate,” the FDA advises. “If a medication has been recalled, pharmacists may be able to dispense the same medication from a manufacturing lot that has not been recalled.
Carcinogen Fibromyalgia Anxiety Disorder Neuropathic Pain Pain Acute Pain Complementary And Alternative Medicine Alternative Treatment Alternative Medicine Complementary Medicine Alternate Medical Therapy Alternate Medicine Skeletal Myoblasts Skeletal Muscle Cancer Pain Pain From Cancer Cancer-Related Pain Diabetic Peripheral Neuropathy Cancer Risk
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Antidepressant recall: FDA upgrades medication risk class to 2nd highest level in recall updateThe U.S. Food and Drug Administration is urging patients not to stop taking their prescribed medication if it falls under the recall but instead to address any concerns and other potential treatment options with their health care provider
Antidepressant recall: FDA upgrades medication risk class to 2nd highest level in recall updateThe U.S. Food and Drug Administration is urging patients not to stop taking their prescribed medication if it falls under the recall but instead to address any concerns and other potential treatment options with their health care provider
Read more »
 FDA announces recall of dipping sauce due to 'potential mold growth contamination'Lunds & Byerlys Lone Star Dip products are being recalled over 'potential mold growth contamination,' according to the FDA. 500 containers sold near the Twin Cities are potentially impacted.
FDA announces recall of dipping sauce due to 'potential mold growth contamination'Lunds & Byerlys Lone Star Dip products are being recalled over 'potential mold growth contamination,' according to the FDA. 500 containers sold near the Twin Cities are potentially impacted.
Read more »
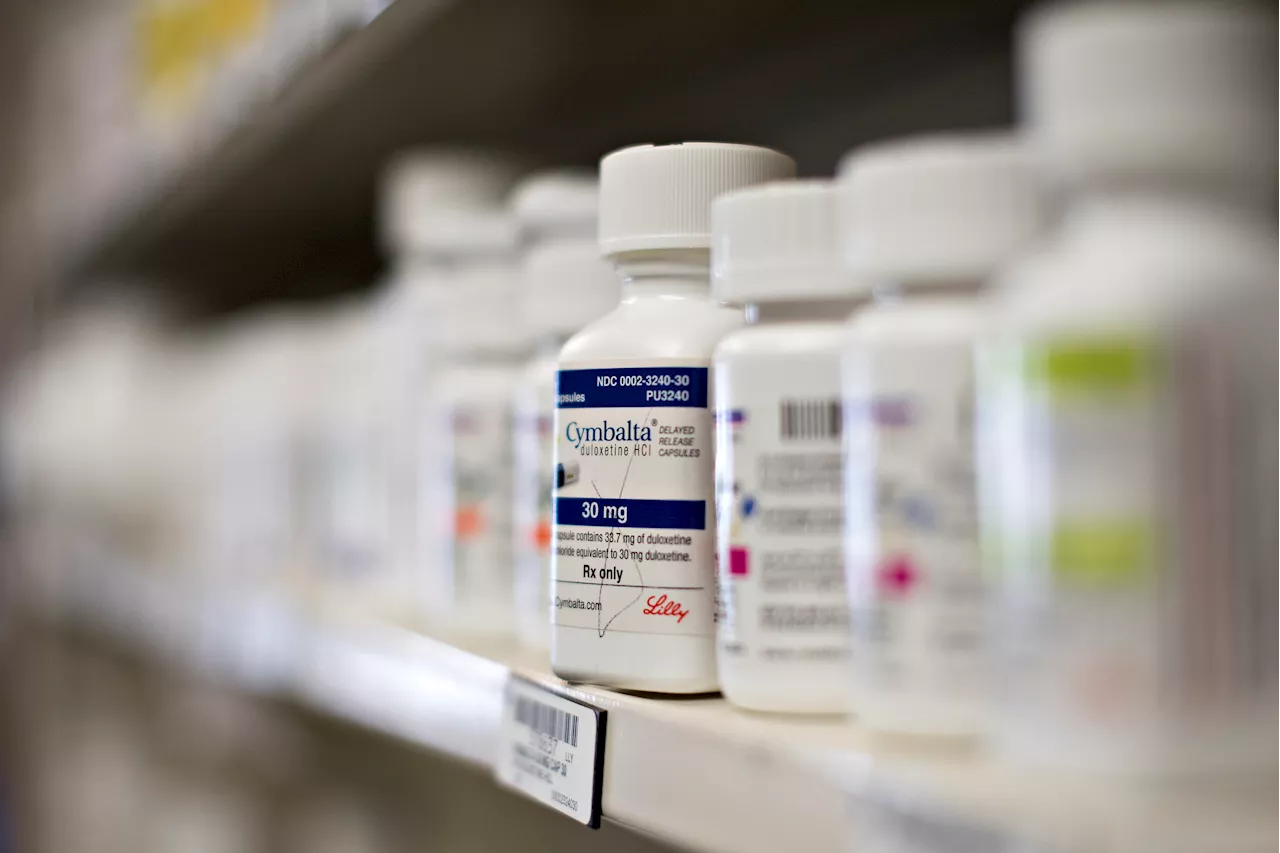 FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
Read more »
 FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
Read more »
 FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
Read more »
 FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
FDA recalls more than 7,000 bottles of antidepressant duloxetine over toxic chemicalThe FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
Read more »
