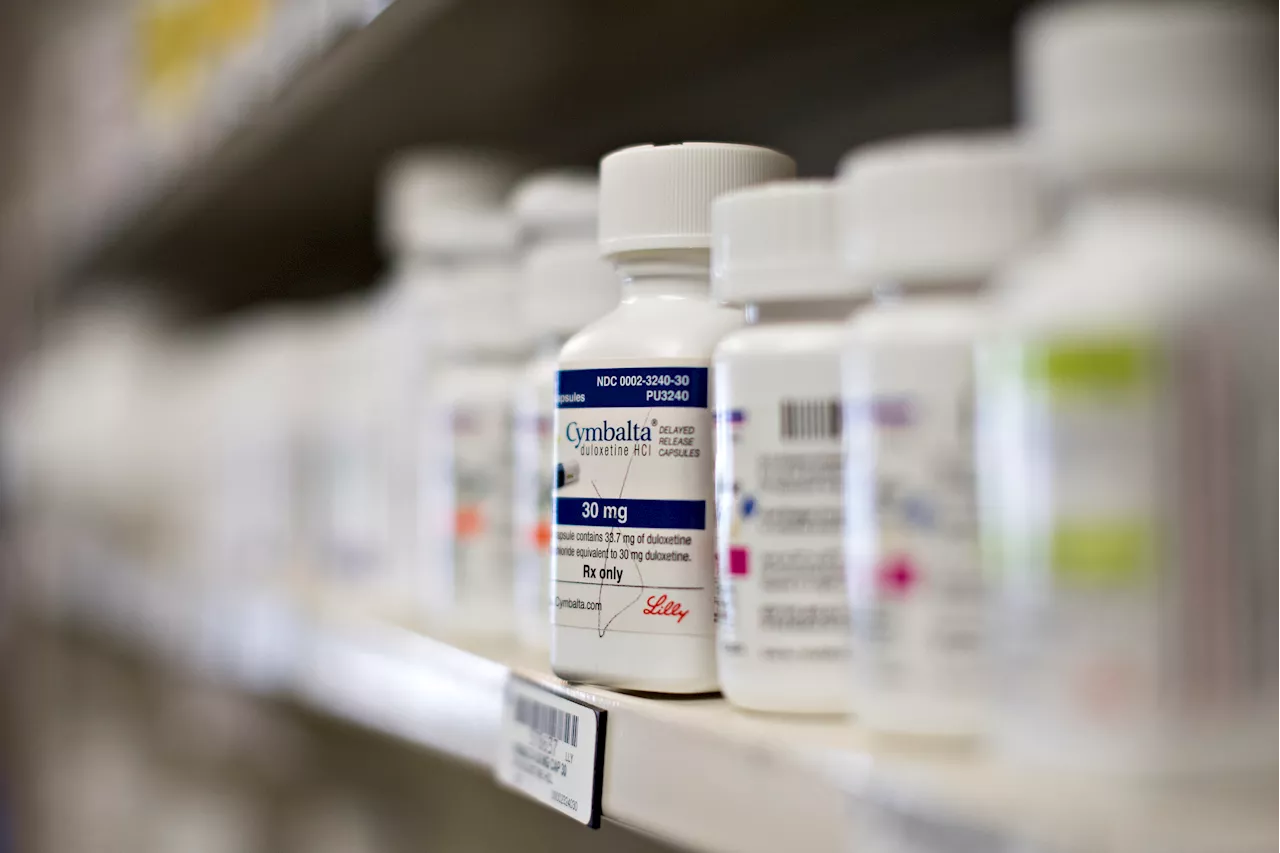
The FDA recalled more than 7,000 bottles of duloxetine, which is sold under the brand name Cymbalta, over the presence of a toxic chemical.
from the agency, the recall involves 7,107 bottles of duloxetine, which is sold under the brand name Cymbalta. Duloxetine is part of a class of drugs called selective serotonin/norepinephrine reuptake inhibitors , which are used to treat depression, anxiety and other mood disorders,The FDA issued the recall due to the presence of N-nitroso-duloxetine above the proposed interim limit.
The recalled capsules are 20mg in strength and sold in 500-count bottles. The lot number for the recalled products is 220128 with an expiration date of December 2024 and manufactured by Towa Pharmaceutical Europe, according to the FDA.
The FDA did not issue specific guidance on what to do with the recalled duloxetine. Patients who take this medication should contact their health care provider.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Thousands of bottles of common antidepressant Cymbalta recalled by FDA over cancer-causing chemicalToday's Business Headlines: 10/22/24
Thousands of bottles of common antidepressant Cymbalta recalled by FDA over cancer-causing chemicalToday's Business Headlines: 10/22/24
Read more »
 FDA Recalls Thousands of Bottles of Popular Antidepressant Cymbalta (Duloxetine)7,100 bottles of Duloxetine, brand name Cymbalta, have been recalled for potential toxic chemical contamination. Find your lot number here.
FDA Recalls Thousands of Bottles of Popular Antidepressant Cymbalta (Duloxetine)7,100 bottles of Duloxetine, brand name Cymbalta, have been recalled for potential toxic chemical contamination. Find your lot number here.
Read more »
 Thousands of duloxetine bottles, an antidepressant sold as Cymbalta, recalled over toxic chemicalSome bottles of the antidepressant duloxetine, sold under the brand name Cymbalta, were recalled due to the presence of a toxic chemical.
Thousands of duloxetine bottles, an antidepressant sold as Cymbalta, recalled over toxic chemicalSome bottles of the antidepressant duloxetine, sold under the brand name Cymbalta, were recalled due to the presence of a toxic chemical.
Read more »
 FDA recalls popular antidepressant due to possible toxic chemical contaminationThe Food and Drug Administration has issued a recall for more than 7,000 bottles of an antidepressant drug because of the presence of a possible cancer-causing chemical.
FDA recalls popular antidepressant due to possible toxic chemical contaminationThe Food and Drug Administration has issued a recall for more than 7,000 bottles of an antidepressant drug because of the presence of a possible cancer-causing chemical.
Read more »
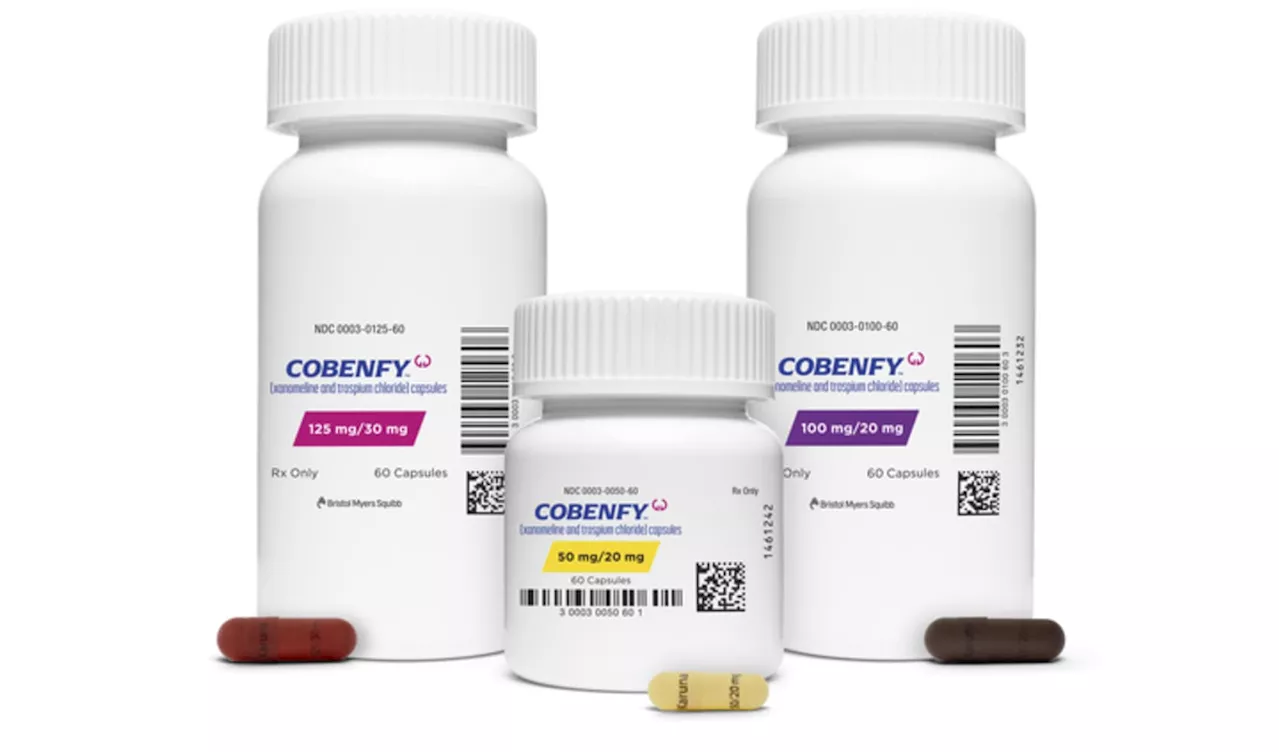 FDA Approves Cobenfy, First Novel Schizophrenia Drug in DecadesThe FDA has approved Cobenfy (cobrenify), a new twice-daily pill developed by Bristol Myers Squibb, as the first novel treatment option for schizophrenia in over 70 years. The medication is expected to be available in late October with a monthly cost of $1,850.
FDA Approves Cobenfy, First Novel Schizophrenia Drug in DecadesThe FDA has approved Cobenfy (cobrenify), a new twice-daily pill developed by Bristol Myers Squibb, as the first novel treatment option for schizophrenia in over 70 years. The medication is expected to be available in late October with a monthly cost of $1,850.
Read more »
 Pennsylvania Woman Receives First FDA-Approved Alzheimer's TreatmentKaren Chung, a 63-year-old woman from Chester Springs, Pennsylvania, has become one of the first patients in the country to receive Kisunla, Eli Lilly and Company's new FDA-approved treatment for early symptomatic Alzheimer's disease. Chung expressed her excitement about receiving the treatment at Abington Neurological Associates, a leading neurology practice.
Pennsylvania Woman Receives First FDA-Approved Alzheimer's TreatmentKaren Chung, a 63-year-old woman from Chester Springs, Pennsylvania, has become one of the first patients in the country to receive Kisunla, Eli Lilly and Company's new FDA-approved treatment for early symptomatic Alzheimer's disease. Chung expressed her excitement about receiving the treatment at Abington Neurological Associates, a leading neurology practice.
Read more »