Scientists make cancer breakthrough that could improve immunotherapy success rate aacr
Myofibroblastic cancer-associated fibroblast –rich tumors generally contain few T cells and respond poorly to immune-checkpoint blockade. Although myoCAFs are associated with poor outcome in most solid tumors, the molecular mechanisms regulating myoCAF accumulation remain unclear, limiting the potential for therapeutic intervention. Here, we identify ataxia-telangiectasia mutated as a central regulator of the myoCAF phenotype.
ATM activation was regulated by the reactive oxygen species–producing enzyme NOX4, both through DNA damage and increased oxidative stress. Targeting fibroblast ATMsuppressed myoCAF-rich tumor growth, promoted intratumoral CD8 T-cell infiltration, and potentiated the response to anti–PD-1 blockade and antitumor vaccination. This work identifies a novel pathway regulating myoCAF differentiation and provides a rationale for using ATM inhibitors to overcome CAF-mediated immunotherapy resistance.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
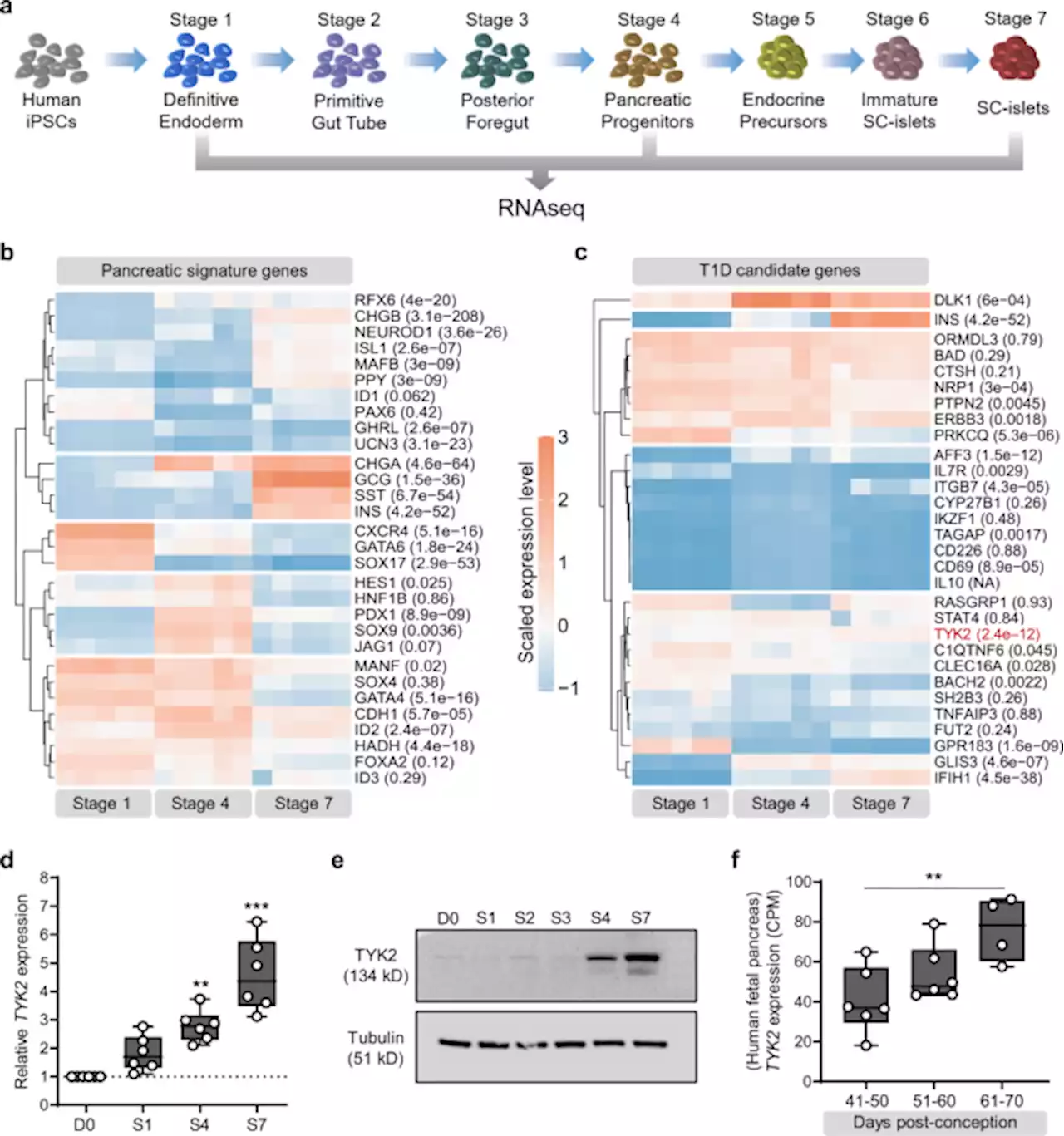 The type 1 diabetes gene TYK2 regulates β-cell development and its responses to interferon-α - Nature CommunicationsThe TYK2 gene is associated with development of type 1 diabetes. Here the authors show that TYK2 regulates β-cell development, but at the same time TYK2 inhibition in the islets prevents IFNα responses and enhances their survival against CD8+ T-cell cytotoxicity; representing a potent therapeutic target to halt T1D progression.
The type 1 diabetes gene TYK2 regulates β-cell development and its responses to interferon-α - Nature CommunicationsThe TYK2 gene is associated with development of type 1 diabetes. Here the authors show that TYK2 regulates β-cell development, but at the same time TYK2 inhibition in the islets prevents IFNα responses and enhances their survival against CD8+ T-cell cytotoxicity; representing a potent therapeutic target to halt T1D progression.
Read more »
 Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: a preclinical evaluation as a novel compound - European Journal of Nuclear Medicine and Molecular ImagingPurpose Targeted α-therapy (TAT) for prostate-specific membrane antigen (PSMA) is a promising treatment for metastatic castration-resistant prostate cancer (CRPC). Astatine is an α-emitter (half-life=7.2 h) that can be produced by a 30-MeV cyclotron. This study evaluated the treatment effect of 211At-labeled PSMA compounds in mouse xenograft models. Methods Tumor xenograft models were established by subcutaneous transplantation of human prostate cancer cells (LNCaP) in NOD/SCID mouse. [211At]PSMA1, [211At]PSMA5, or [211At]PSMA6 was administered to LNCaP xenograft mice to evaluate biodistribution at 3 and 24 h. The treatment effect was evaluated by administering [211At]PSMA1 (0.40 ± 0.07 MBq), [211At]PSMA5 (0.39 ± 0.03 MBq), or saline. Histopathological evaluation was performed for the at-risk organs at 3 and 6 weeks after administration. Results [211At]PSMA5 resulted in higher tumor retention compared to [211At]PSMA1 and [211At]PSMA6 (30.6 ± 17.8, 12.4 ± 4.8, and 19.1 ± 4.5 %ID/g at 3 h versus 40.7 ± 2.6, 8.7 ± 3.5, and 18.1 ± 2.2%ID/g at 24 h, respectively), whereas kidney excretion was superior in [211At]PSMA1 compared to [211At]PSMA5 and [211At]PSMA6. An excellent treatment effect on tumor growth was observed after [211At]PSMA5 administration. [211At]PSMA1 also showed a substantial treatment effect; however, the tumor size was relatively larger compared to that with [211At]PSMA5. In the histopathological evaluation, regenerated tubules were detected in the kidneys at 3 and 6 weeks after the administration of [211At]PSMA5. Conclusion TAT using [211At]PSMA5 resulted in excellent tumor growth suppression with minimal side effects in the normal organs. [211At]PSMA5 should be considered a new possible TAT for metastatic CRPC, and translational prospective trials are warranted.
Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: a preclinical evaluation as a novel compound - European Journal of Nuclear Medicine and Molecular ImagingPurpose Targeted α-therapy (TAT) for prostate-specific membrane antigen (PSMA) is a promising treatment for metastatic castration-resistant prostate cancer (CRPC). Astatine is an α-emitter (half-life=7.2 h) that can be produced by a 30-MeV cyclotron. This study evaluated the treatment effect of 211At-labeled PSMA compounds in mouse xenograft models. Methods Tumor xenograft models were established by subcutaneous transplantation of human prostate cancer cells (LNCaP) in NOD/SCID mouse. [211At]PSMA1, [211At]PSMA5, or [211At]PSMA6 was administered to LNCaP xenograft mice to evaluate biodistribution at 3 and 24 h. The treatment effect was evaluated by administering [211At]PSMA1 (0.40 ± 0.07 MBq), [211At]PSMA5 (0.39 ± 0.03 MBq), or saline. Histopathological evaluation was performed for the at-risk organs at 3 and 6 weeks after administration. Results [211At]PSMA5 resulted in higher tumor retention compared to [211At]PSMA1 and [211At]PSMA6 (30.6 ± 17.8, 12.4 ± 4.8, and 19.1 ± 4.5 %ID/g at 3 h versus 40.7 ± 2.6, 8.7 ± 3.5, and 18.1 ± 2.2%ID/g at 24 h, respectively), whereas kidney excretion was superior in [211At]PSMA1 compared to [211At]PSMA5 and [211At]PSMA6. An excellent treatment effect on tumor growth was observed after [211At]PSMA5 administration. [211At]PSMA1 also showed a substantial treatment effect; however, the tumor size was relatively larger compared to that with [211At]PSMA5. In the histopathological evaluation, regenerated tubules were detected in the kidneys at 3 and 6 weeks after the administration of [211At]PSMA5. Conclusion TAT using [211At]PSMA5 resulted in excellent tumor growth suppression with minimal side effects in the normal organs. [211At]PSMA5 should be considered a new possible TAT for metastatic CRPC, and translational prospective trials are warranted.
Read more »
 Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancerAbstract. Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer death. Despite initial responses to intervention, up to 80% of patient tumors recur and require additional treatment. Retrospective clinical analysis of OC patients indicates antibiotic use during chemotherapy treatment is associated with poor overall survival. Here, we assessed whether antibiotic (ABX) treatment would impact growth of EOC and sensitivity to cisplatin. Immunocompetent or immunocompromised mice were given untreated control or ABX-containing (metronidazole, ampicillin, vancomycin, and neomycin) water prior to intraperitoneal injection with EOC cells, and cisplatin therapy was administered biweekly until endpoint. Tumor-bearing ABX-treated mice exhibited accelerated tumor growth and resistance to cisplatin therapy compared with control treatment. ABX treatment led to reduced apoptosis, increased DNA damage repair, and enhanced angiogenesis in cisplatin-treated tumors, and tumors from ABX-treated mice contained a higher frequency of cisplatin-augmented cancer stem cells than control mice. Stool analysis indicated non-resistant gut microbial species were disrupted by ABX treatment. Cecal transplants of microbiota derived from control-treated mice was sufficient to ameliorate chemoresistance and prolong survival of ABX-treated mice, indicative of a gut-derived tumor suppressor. Metabolomics analyses identified circulating gut-derived metabolites that were altered by ABX treatment and restored by recolonization, providing candidate metabolites that mediate the crosstalk between the gut microbiome and ovarian cancer. Collectively, these findings indicate that an intact microbiome functions as a tumor suppressor in EOC, and perturbation of the gut microbiota with ABX treatment promotes tumor growth and suppresses cisplatin sensitivity.
Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancerAbstract. Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer death. Despite initial responses to intervention, up to 80% of patient tumors recur and require additional treatment. Retrospective clinical analysis of OC patients indicates antibiotic use during chemotherapy treatment is associated with poor overall survival. Here, we assessed whether antibiotic (ABX) treatment would impact growth of EOC and sensitivity to cisplatin. Immunocompetent or immunocompromised mice were given untreated control or ABX-containing (metronidazole, ampicillin, vancomycin, and neomycin) water prior to intraperitoneal injection with EOC cells, and cisplatin therapy was administered biweekly until endpoint. Tumor-bearing ABX-treated mice exhibited accelerated tumor growth and resistance to cisplatin therapy compared with control treatment. ABX treatment led to reduced apoptosis, increased DNA damage repair, and enhanced angiogenesis in cisplatin-treated tumors, and tumors from ABX-treated mice contained a higher frequency of cisplatin-augmented cancer stem cells than control mice. Stool analysis indicated non-resistant gut microbial species were disrupted by ABX treatment. Cecal transplants of microbiota derived from control-treated mice was sufficient to ameliorate chemoresistance and prolong survival of ABX-treated mice, indicative of a gut-derived tumor suppressor. Metabolomics analyses identified circulating gut-derived metabolites that were altered by ABX treatment and restored by recolonization, providing candidate metabolites that mediate the crosstalk between the gut microbiome and ovarian cancer. Collectively, these findings indicate that an intact microbiome functions as a tumor suppressor in EOC, and perturbation of the gut microbiota with ABX treatment promotes tumor growth and suppresses cisplatin sensitivity.
Read more »
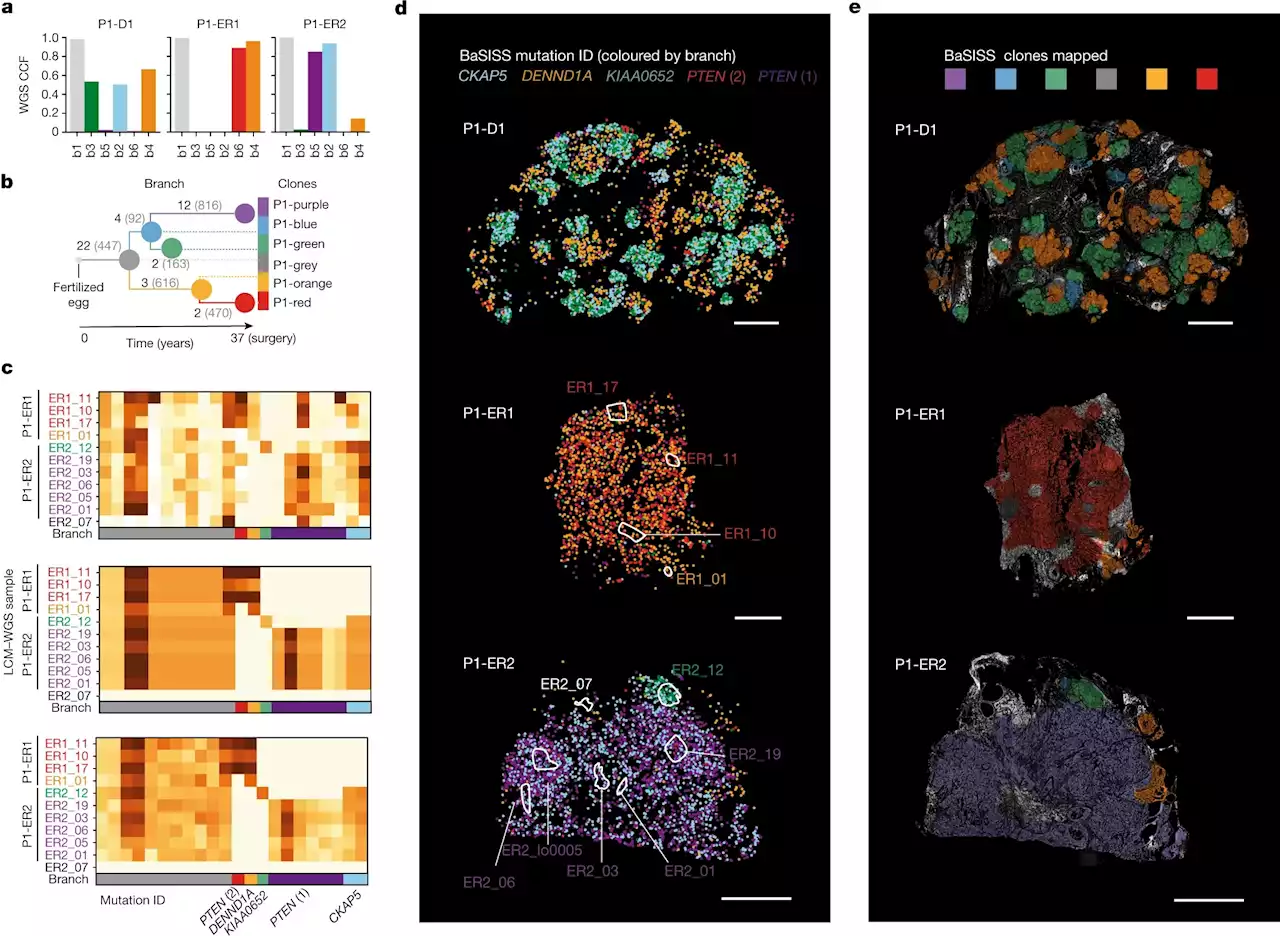 Breast cancer spread uncovered by new molecular microscopyNew technology can trace which populations of breast cancer cells are responsible for the spread of the disease, and for the first time highlights how the location of cancer cells could be as important as mutations in tumor growth.
Breast cancer spread uncovered by new molecular microscopyNew technology can trace which populations of breast cancer cells are responsible for the spread of the disease, and for the first time highlights how the location of cancer cells could be as important as mutations in tumor growth.
Read more »
 Deficiency for SAMHD1 activates MDA5 in a cGAS/STING-dependent mannerLoss of the dNTPase and DNA repair enzyme SAMHD1 is associated with cancer and causes systemic autoimmunity. We show transformation-promoting spontaneous DNA da
Deficiency for SAMHD1 activates MDA5 in a cGAS/STING-dependent mannerLoss of the dNTPase and DNA repair enzyme SAMHD1 is associated with cancer and causes systemic autoimmunity. We show transformation-promoting spontaneous DNA da
Read more »
 Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancerAbstract. Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer death. Despite initial responses to intervention, up to 80% of patient tumors recur and require additional treatment. Retrospective clinical analysis of OC patients indicates antibiotic use during chemotherapy treatment is associated with poor overall survival. Here, we assessed whether antibiotic (ABX) treatment would impact growth of EOC and sensitivity to cisplatin. Immunocompetent or immunocompromised mice were given untreated control or ABX-containing (metronidazole, ampicillin, vancomycin, and neomycin) water prior to intraperitoneal injection with EOC cells, and cisplatin therapy was administered biweekly until endpoint. Tumor-bearing ABX-treated mice exhibited accelerated tumor growth and resistance to cisplatin therapy compared with control treatment. ABX treatment led to reduced apoptosis, increased DNA damage repair, and enhanced angiogenesis in cisplatin-treated tumors, and tumors from ABX-treated mice contained a higher frequency of cisplatin-augmented cancer stem cells than control mice. Stool analysis indicated non-resistant gut microbial species were disrupted by ABX treatment. Cecal transplants of microbiota derived from control-treated mice was sufficient to ameliorate chemoresistance and prolong survival of ABX-treated mice, indicative of a gut-derived tumor suppressor. Metabolomics analyses identified circulating gut-derived metabolites that were altered by ABX treatment and restored by recolonization, providing candidate metabolites that mediate the crosstalk between the gut microbiome and ovarian cancer. Collectively, these findings indicate that an intact microbiome functions as a tumor suppressor in EOC, and perturbation of the gut microbiota with ABX treatment promotes tumor growth and suppresses cisplatin sensitivity.
Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancerAbstract. Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer death. Despite initial responses to intervention, up to 80% of patient tumors recur and require additional treatment. Retrospective clinical analysis of OC patients indicates antibiotic use during chemotherapy treatment is associated with poor overall survival. Here, we assessed whether antibiotic (ABX) treatment would impact growth of EOC and sensitivity to cisplatin. Immunocompetent or immunocompromised mice were given untreated control or ABX-containing (metronidazole, ampicillin, vancomycin, and neomycin) water prior to intraperitoneal injection with EOC cells, and cisplatin therapy was administered biweekly until endpoint. Tumor-bearing ABX-treated mice exhibited accelerated tumor growth and resistance to cisplatin therapy compared with control treatment. ABX treatment led to reduced apoptosis, increased DNA damage repair, and enhanced angiogenesis in cisplatin-treated tumors, and tumors from ABX-treated mice contained a higher frequency of cisplatin-augmented cancer stem cells than control mice. Stool analysis indicated non-resistant gut microbial species were disrupted by ABX treatment. Cecal transplants of microbiota derived from control-treated mice was sufficient to ameliorate chemoresistance and prolong survival of ABX-treated mice, indicative of a gut-derived tumor suppressor. Metabolomics analyses identified circulating gut-derived metabolites that were altered by ABX treatment and restored by recolonization, providing candidate metabolites that mediate the crosstalk between the gut microbiome and ovarian cancer. Collectively, these findings indicate that an intact microbiome functions as a tumor suppressor in EOC, and perturbation of the gut microbiota with ABX treatment promotes tumor growth and suppresses cisplatin sensitivity.
Read more »
