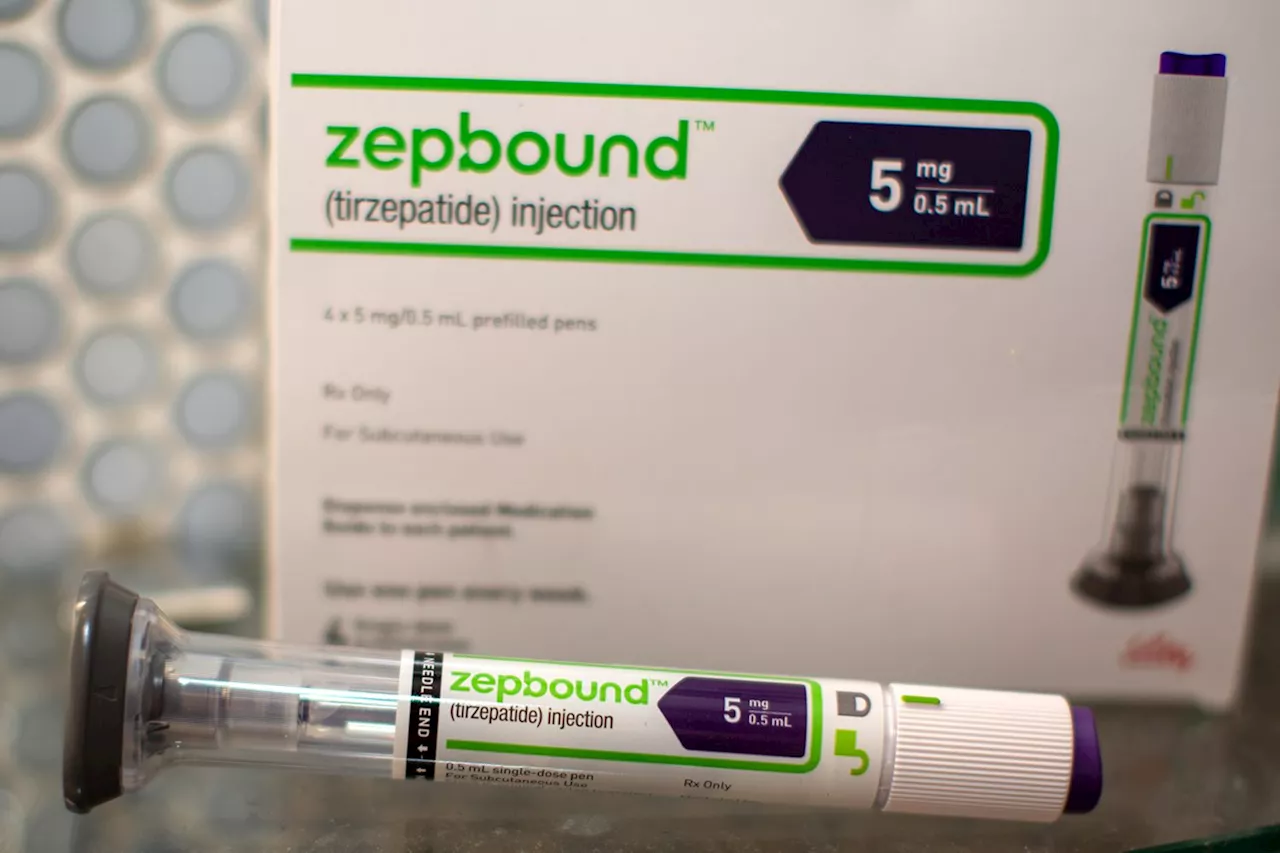
The FDA has approved the use of the weight loss drug Zepbound (tirzepatide) to treat moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
Charna Flam is a writer-reporter at PEOPLE. She has been working at PEOPLE since 2023. Her work has previously appeared on Variety, The New York Post, and The Wrap.The weight loss drug Zepbound , generically known as tirzepatide, is also now an approved medication to treat obstructive sleep apnea , per a Food and Drug Administration Dec. 20The federal agency authorized the use of the Eli Lilly & Co.
weight loss drug for “treatment of moderate to severe obstructive sleep apnea (OSA) in adults with obesity.” In addition to the GLP-1 prescription, the FDA suggests that users reduce their calorie intake and increase their exercise. Sleep apnea “occurs when a person’s upper airway becomes blocked, causing pauses in breathing during sleep,” per the FDA. “Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said Sally Seymour, M.D., director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research, per the press release. “This is a major step forward for patients with obstructive sleep apnea.”The FDA conducted two studies, both of which found that Zepbound helps reduce sleep apnea symptoms in some patients by aiding weight loss. Both studies monitored obese adults with moderate to severe sleep apnea over a 52-week period. Unlike the participants who received the placebo, those who received the drug experienced a 'statistically significant and clinically meaningful reduction' in episodes of shallow breathing or temporary pauses in breathing. This finding was found in both participants who used a CPAP (continuous positive airway pressure) machine and those who didn’t.supports the FDA’s new authorization . However, the organization, which is “dedicated exclusively to the medical subspecialty of sleep medicine,” said Zepbound as a sleep apnea treatment is not an option for everyon
FDA Sleep Apnea Weight Loss Drug Zepbound Tirzepatide
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves weight loss drug Zepbound to treat obstructive sleep apneaThe FDA said studies have shown that by aiding weight loss, Zepbound improves sleep apnea symptoms in some patients.
FDA approves weight loss drug Zepbound to treat obstructive sleep apneaThe FDA said studies have shown that by aiding weight loss, Zepbound improves sleep apnea symptoms in some patients.
Read more »
 FDA approves weight loss drug Zepbound to treat obstructive sleep apneaThe FDA said studies have shown that by aiding weight loss, Zepbound improves sleep apnea symptoms in some patients.
FDA approves weight loss drug Zepbound to treat obstructive sleep apneaThe FDA said studies have shown that by aiding weight loss, Zepbound improves sleep apnea symptoms in some patients.
Read more »
 Weight Loss Drug Zepbound Approved to Treat Sleep ApneaThe FDA expanded the approval of weight loss drug Zepbound to treat obstructive sleep apnea in adults with obesity. This makes it the first drug treatment for the disorder, which affects about 39 million adults in the United States.
Weight Loss Drug Zepbound Approved to Treat Sleep ApneaThe FDA expanded the approval of weight loss drug Zepbound to treat obstructive sleep apnea in adults with obesity. This makes it the first drug treatment for the disorder, which affects about 39 million adults in the United States.
Read more »
 FDA Approves Weight Loss Drug Zepbound for Sleep ApneaThe FDA has granted approval to Zepbound, a popular weight loss medication, for the treatment of moderate to severe obstructive sleep apnea. This marks the first FDA-approved prescription drug specifically for adults with obstructive sleep apnea.
FDA Approves Weight Loss Drug Zepbound for Sleep ApneaThe FDA has granted approval to Zepbound, a popular weight loss medication, for the treatment of moderate to severe obstructive sleep apnea. This marks the first FDA-approved prescription drug specifically for adults with obstructive sleep apnea.
Read more »
 FDA Approves Weight-Loss Drug Zepbound for Sleep ApneaIt’s the first prescription drug to be approved to treat the condition.
FDA Approves Weight-Loss Drug Zepbound for Sleep ApneaIt’s the first prescription drug to be approved to treat the condition.
Read more »
 FDA approves Eli Lilly's weight loss drug Zepbound for sleep apnea, expanding use in U.S.The agency’s decision expands the use of Zepbound and could potentially pave the way for Eli Lilly to gain broader insurance coverage for the treatment.
FDA approves Eli Lilly's weight loss drug Zepbound for sleep apnea, expanding use in U.S.The agency’s decision expands the use of Zepbound and could potentially pave the way for Eli Lilly to gain broader insurance coverage for the treatment.
Read more »