Vertex Pharmaceuticals' non-opioid pain medication, suzetrigine (Journavx), has received FDA approval for treating moderate to severe acute pain in adults. This groundbreaking drug offers a promising alternative to opioids, targeting a pain-signaling pathway in the peripheral nervous system.
Vertex Pharmaceuticals has achieved a significant milestone with the Food and Drug Administration (FDA) approving its non-opioid pain medication , suzetrigine, for use in adults. This groundbreaking drug, marketed under the brand name Journavx , targets a pain-signaling pathway involving sodium channels in the peripheral nervous system, effectively preventing pain signals from reaching the brain. Journavx received the green light for treating moderate to severe acute pain in adults on Thursday.
The company's CEO, Reshma Kewalramani, hailed Journavx as the 'first new class of pain medicine approved in more than 20 years,' emphasizing its potential to revolutionize acute pain management and establish a new standard of care. In clinical trials involving patients who underwent abdominoplasties and bunionectomies, Journavx demonstrated a 'statistically significant' reduction in pain compared to a placebo within two days. These trials were meticulously designed as randomized, double-blind, placebo-controlled, and active-controlled studies. The FDA, recognizing the significance of this new therapeutic option, emphasized its commitment to approving safe and effective alternatives to opioids for pain management. Acting Director of the FDA Center for Drug Evaluation and Research, Dr. Jacqueline Corrigan-Curay, stated that 'A new non-opioid analgesic therapeutic class for acute pain offers an opportunity to mitigate certain risks associated with using an opioid for pain and provides patients with another treatment option.'Vertex highlights that a substantial proportion of patients initially prescribed opioids for acute pain end up enduring prolonged opioid use, which can lead to addiction. Journavx's wholesale per-tablet acquisition cost is set at $15.50. The FDA has issued a warning that suzetrigine is 'contraindicated' for individuals using 'strong CYP3A inhibitors' and advises against consuming grapefruit or grapefruit-containing products while taking the medication. Following the FDA approval announcement, Vertex experienced a surge in its stock price, with shares jumping over 5% on Friday. The company's market capitalization reached approximately $119.81 billion in the afternoon. Vertex, established in the late 1980s, currently boasts six FDA-approved drugs, according to its website. The company is actively exploring the potential applications of Journavx for treating peripheral neuropathic pain
Non-Opioid Pain Medication FDA Approval Journavx Vertex Pharmaceuticals Acute Pain Opioid Alternative Suzetrigine
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Approves Vertex's Non-Opioid Painkiller Journavx, Offering Hope for Millions Suffering from PainVertex Pharmaceuticals' non-opioid painkiller, Journavx, receives FDA approval, marking a significant milestone in the fight against the opioid epidemic. The drug offers a safe and effective alternative for pain relief without the risk of addiction.
FDA Approves Vertex's Non-Opioid Painkiller Journavx, Offering Hope for Millions Suffering from PainVertex Pharmaceuticals' non-opioid painkiller, Journavx, receives FDA approval, marking a significant milestone in the fight against the opioid epidemic. The drug offers a safe and effective alternative for pain relief without the risk of addiction.
Read more »
 FDA Approves Vertex Pharmaceuticals' Non-Opioid Painkiller JournavxThe Food and Drug Administration (FDA) has approved Vertex Pharmaceuticals' non-opioid painkiller, Journavx, marking a significant step forward in safer pain management. This milestone comes after years of efforts to develop alternatives to addictive opioids. Journavx works by blocking pain signals at their source, unlike opioids which directly impact the brain.
FDA Approves Vertex Pharmaceuticals' Non-Opioid Painkiller JournavxThe Food and Drug Administration (FDA) has approved Vertex Pharmaceuticals' non-opioid painkiller, Journavx, marking a significant step forward in safer pain management. This milestone comes after years of efforts to develop alternatives to addictive opioids. Journavx works by blocking pain signals at their source, unlike opioids which directly impact the brain.
Read more »
 FDA Approves New Nonopioid Pain Killer JournavxThe FDA has approved a new nonopioid painkiller, Journavx, for moderate to severe acute pain in adults. This could potentially reduce opioid prescriptions after surgery or provide an alternative for those who can't tolerate other pain medications.
FDA Approves New Nonopioid Pain Killer JournavxThe FDA has approved a new nonopioid painkiller, Journavx, for moderate to severe acute pain in adults. This could potentially reduce opioid prescriptions after surgery or provide an alternative for those who can't tolerate other pain medications.
Read more »
 FDA Approves Vertex's Non-Opioid Painkiller: A New Era in Pain ManagementThe Food and Drug Administration (FDA) has greenlit Vertex Pharmaceutical's non-opioid painkiller, presenting a groundbreaking alternative to traditional opioid-based pain relief options and offering hope in the fight against the opioid epidemic.
FDA Approves Vertex's Non-Opioid Painkiller: A New Era in Pain ManagementThe Food and Drug Administration (FDA) has greenlit Vertex Pharmaceutical's non-opioid painkiller, presenting a groundbreaking alternative to traditional opioid-based pain relief options and offering hope in the fight against the opioid epidemic.
Read more »
 Opioid-Free Pain Relief: FDA Approves Journavx for Acute PainThe FDA has approved suzetrigine (Journavx) for the treatment of moderate to severe pain in adults, offering a new, non-addictive alternative to opioids. This groundbreaking drug targets a specific sodium channel in the peripheral nervous system, effectively blocking pain signals from reaching the brain.
Opioid-Free Pain Relief: FDA Approves Journavx for Acute PainThe FDA has approved suzetrigine (Journavx) for the treatment of moderate to severe pain in adults, offering a new, non-addictive alternative to opioids. This groundbreaking drug targets a specific sodium channel in the peripheral nervous system, effectively blocking pain signals from reaching the brain.
Read more »
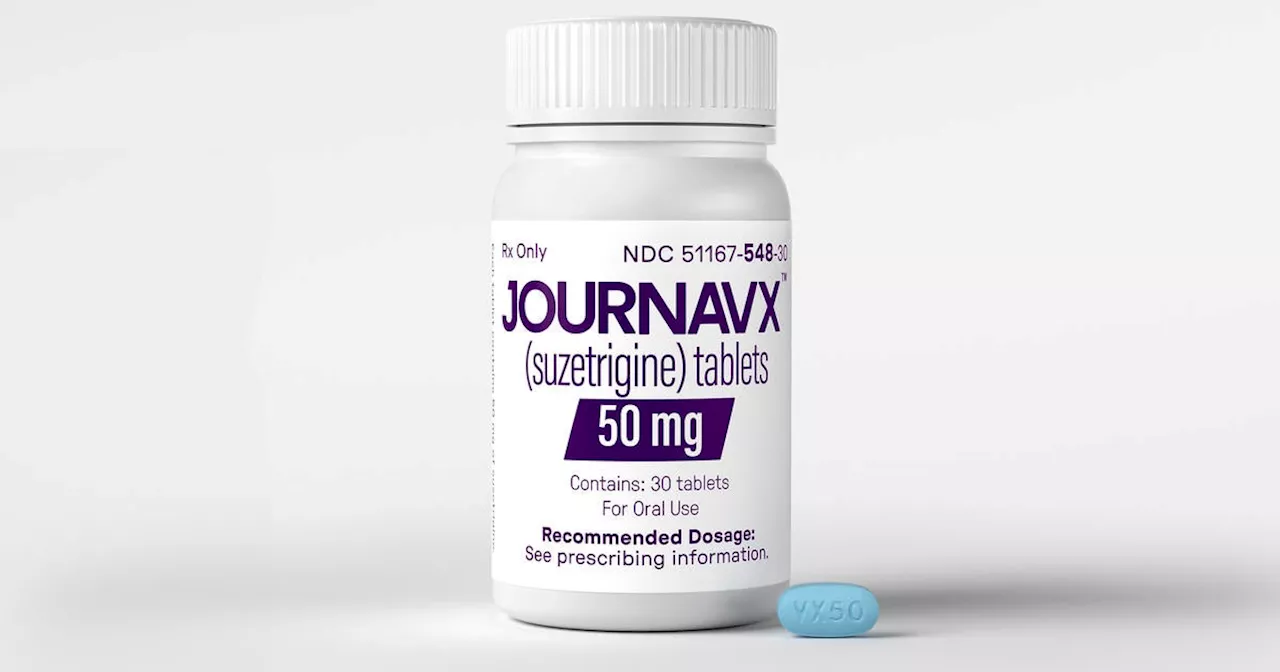 FDA Approves First Novel Pain Drug in Over 20 Years: Journavx Offers Hope for Safer Pain ReliefThe U.S. Food and Drug Administration (FDA) has approved Journavx, a groundbreaking new pain medication developed by Vertex Pharmaceuticals, offering a promising alternative to opioids and over-the-counter painkillers.
FDA Approves First Novel Pain Drug in Over 20 Years: Journavx Offers Hope for Safer Pain ReliefThe U.S. Food and Drug Administration (FDA) has approved Journavx, a groundbreaking new pain medication developed by Vertex Pharmaceuticals, offering a promising alternative to opioids and over-the-counter painkillers.
Read more »
