“It’s going to be completely game changing for a lot of women,” one endocrinologist said.
There may be relief for women who suffer through hot flashes, night sweats, chills and other symptoms of menopause.
The Food and Drug Administration approved a new drug called Veozah , which is a one-of-a-kind treatment for menopause symptoms. As a non-hormonal drug, it’s a boon for women who cannot use hormone-replacement therapy for hot flashes. “It’s going to be completely game changing for a lot of women,” Professor Waljit Dhillo, an endocrinologist at Imperial College London who was involved in early research into Veozah,“It’s like a switch. Within a day or two the flushes go away. It’s unbelievable how well these drugs work,” he added.
Veozah works by blocking the activity of neurokinin 3 , a chemical in the brain. NK3 helps regulate body temperature in the hypothalamus, sometimes called the brain’s “thermostat.”Declining levels of estrogen during menopause disrupt the balance between NK3 and estrogen, causing the hypothalamus to send out signals indicating that the body is overheating, and the result is hot flashes and night sweats.
By blocking the activity of NK3, Veozah makes the hypothalamus stop overreacting, and the hot flashes end. Veozah is the first and only drug that works this way to help with hot flashes,
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
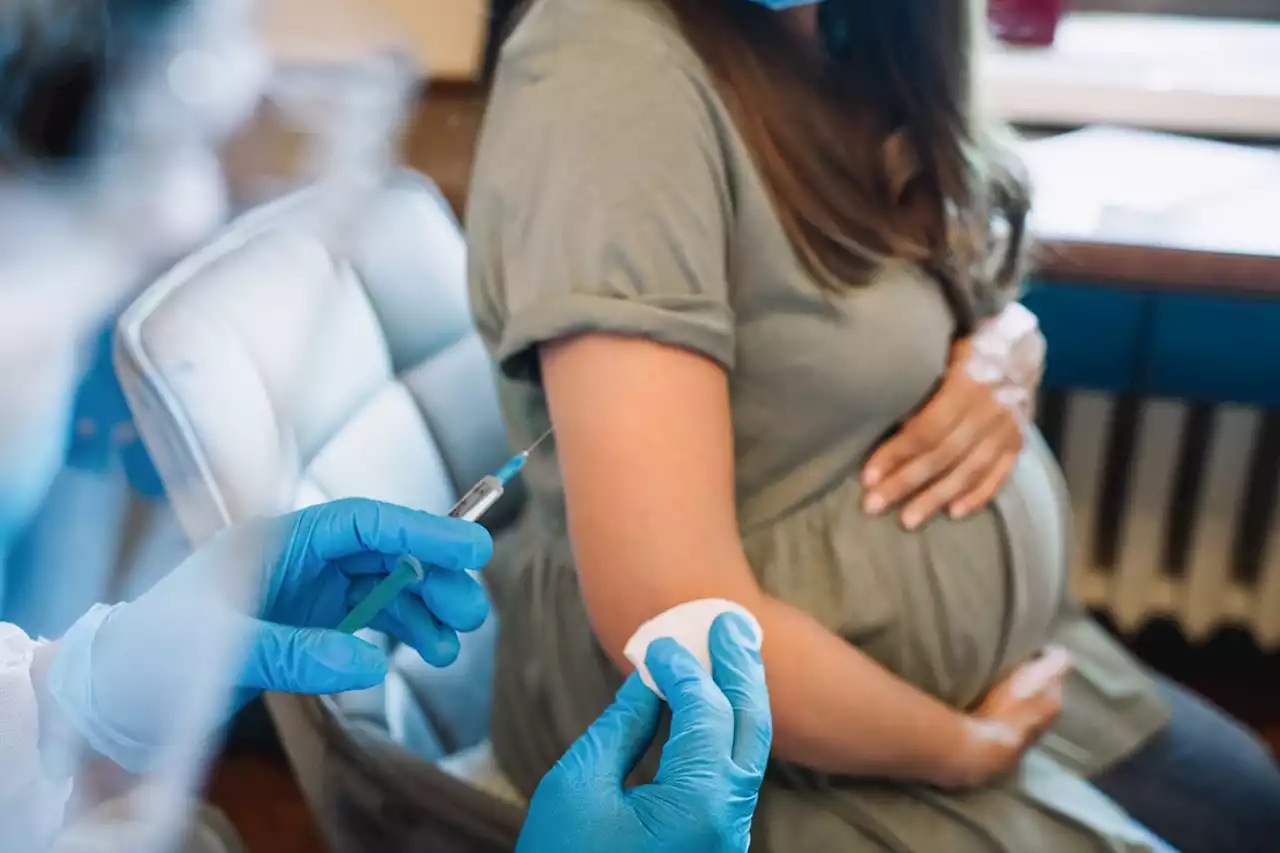 FDA Advisory Panel Recommends RSV Vaccine for Pregnant Women'Before the pandemic, RSV was the No. 1 cause of infant hospitalization in the United States, so this is a big deal.'
FDA Advisory Panel Recommends RSV Vaccine for Pregnant Women'Before the pandemic, RSV was the No. 1 cause of infant hospitalization in the United States, so this is a big deal.'
Read more »
 The End of the FDA’s “Gay Blood Ban” Has a Puzzling, Damaging ExceptionThe agency can't quit blanket bans that target gay and bisexual men.
The End of the FDA’s “Gay Blood Ban” Has a Puzzling, Damaging ExceptionThe agency can't quit blanket bans that target gay and bisexual men.
Read more »
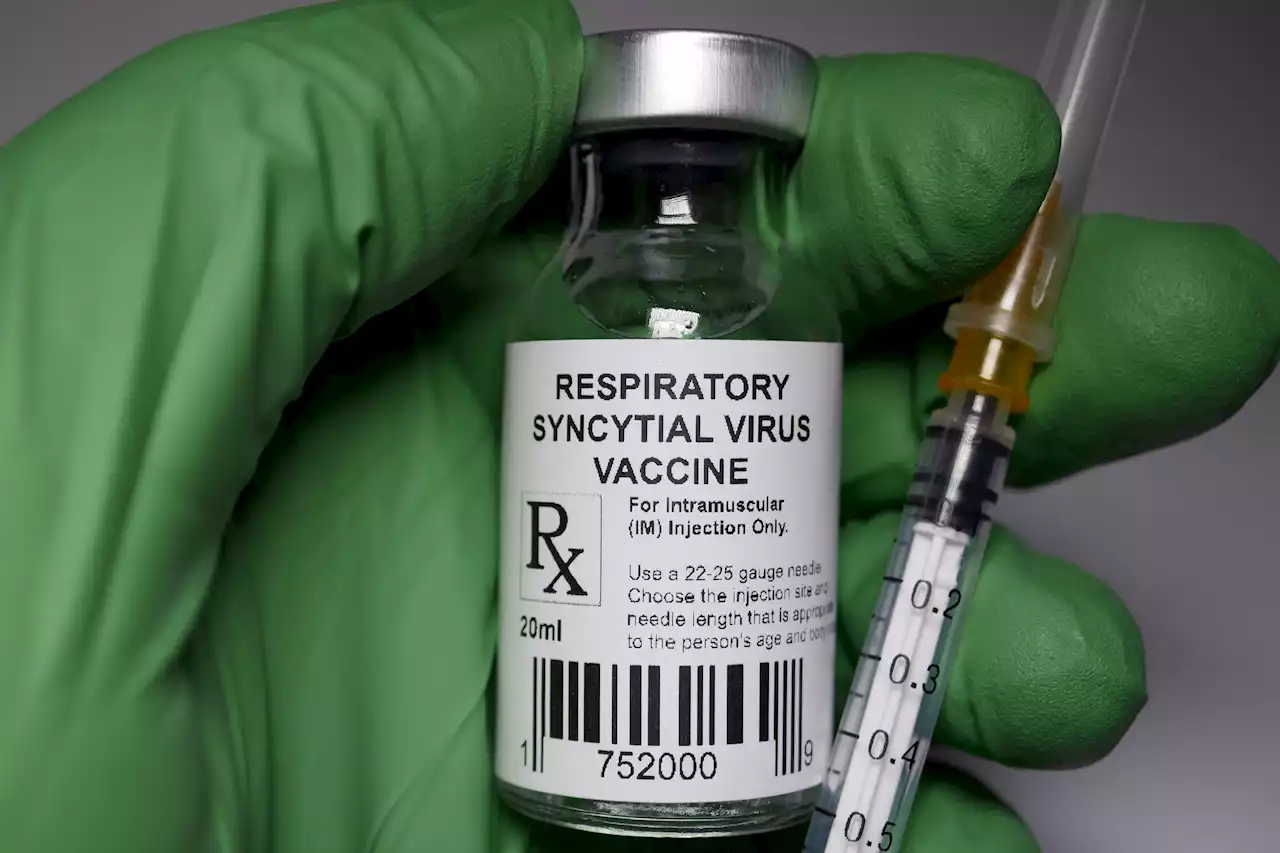 FDA Advisors Recommend Approval for First Vaccine to Protect Babies From RSVThis week, an FDA advisory panel announced their support for approval, voting unanimously on the vaccine’s effectiveness.
FDA Advisors Recommend Approval for First Vaccine to Protect Babies From RSVThis week, an FDA advisory panel announced their support for approval, voting unanimously on the vaccine’s effectiveness.
Read more »
 FDA Approves Autoinjector Pen for Humira Biosimilar, CyltezoThe FDA approved a new autoinjection option for adalimumab-adbm, a biosimilar to AbbVie's adalimumab, ahead of Cyltezo's commercial launch on July 1, 2023.
FDA Approves Autoinjector Pen for Humira Biosimilar, CyltezoThe FDA approved a new autoinjection option for adalimumab-adbm, a biosimilar to AbbVie's adalimumab, ahead of Cyltezo's commercial launch on July 1, 2023.
Read more »
 FDA Approves First Pill to Treat Moderate-to-Severe Crohn's DiseaseRinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
FDA Approves First Pill to Treat Moderate-to-Severe Crohn's DiseaseRinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
Read more »
