The U.S. Food and Drug Administration (FDA) on Friday classified the recall of Getinge AB's heart devices as the most serious type, saying their use may cause injuries or death.
March 17 - The U.S. Food and Drug Administration on Friday classified the recall of Getinge AB'sThe Swedish medical equipment maker's unit, Datascope, recalled an estimated 2,300 devices in the United States in January.
Coiled cable connecting the display and base on some devices of the company may fail, causing an unexpected shutdown, according to the FDA. The devices are designed to help the heart pump more blood and an unexpected pump shutdown and any interruption to therapy can lead to unstable blood flow, organ damage, including death, especially for people who are critically ill.
Datascope has reported 44 complaints about damaged coiled cords resulting in unexpected shutdowns from June 2019 to August 2022, the FDA said, adding that there have been no reports of injuries or deaths related to this issue.Getinge did not immediately respond to a Reuters request for comment.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Food Nonprofit Bread For The City Pausing Food And Social Services For A MonthBread for the City is pausing all programs and services for a month starting Monday to allow for staff “rest, mourning, reflection, and planning.”
Food Nonprofit Bread For The City Pausing Food And Social Services For A MonthBread for the City is pausing all programs and services for a month starting Monday to allow for staff “rest, mourning, reflection, and planning.”
Read more »
 Food Nonprofit Bread For The City Pausing Food Services For A MonthThe local non-profit that provides a variety of food, medical, legal, and social services will be shutting down its food services from March 20 to April 18.
Food Nonprofit Bread For The City Pausing Food Services For A MonthThe local non-profit that provides a variety of food, medical, legal, and social services will be shutting down its food services from March 20 to April 18.
Read more »
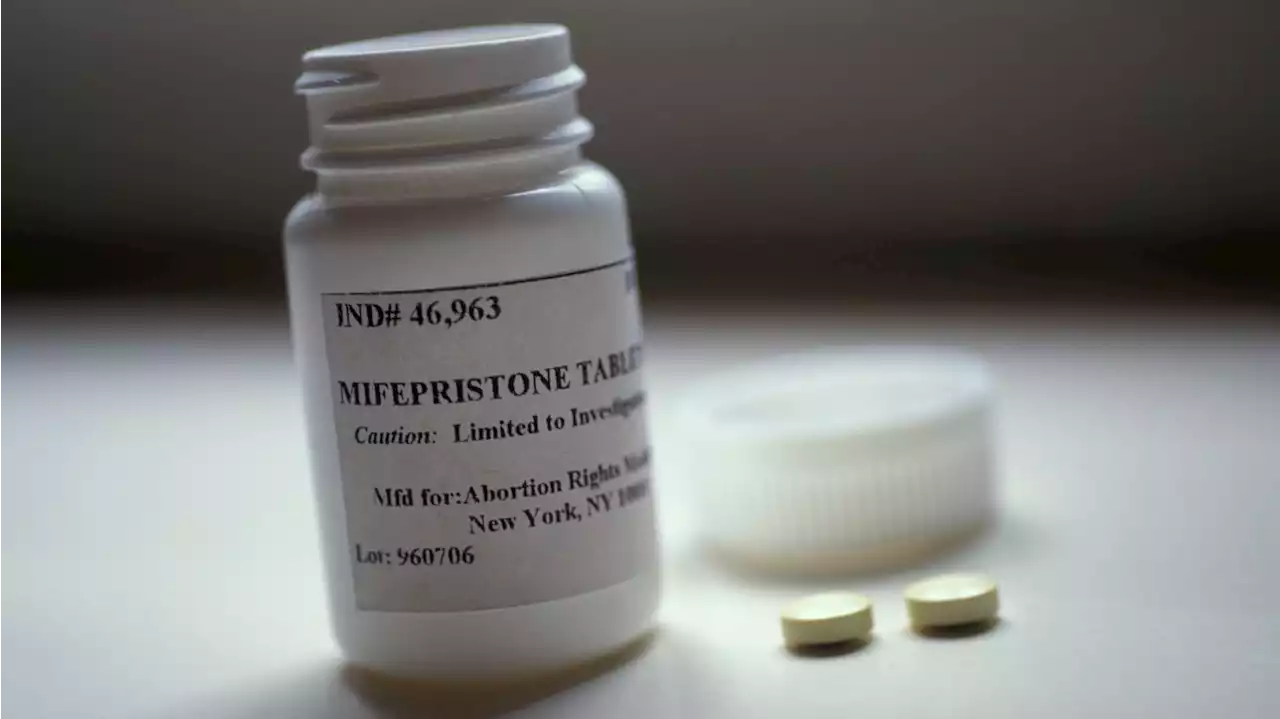 Abortion pill ruling looms over FDA's drug approval processA federal judge in Texas is poised to rule 'as soon as possible' on whether to suspend the FDA's approval of a widely-used abortion pill and potentially reverse the agency's authority on drug regulation for the first time.
Abortion pill ruling looms over FDA's drug approval processA federal judge in Texas is poised to rule 'as soon as possible' on whether to suspend the FDA's approval of a widely-used abortion pill and potentially reverse the agency's authority on drug regulation for the first time.
Read more »
 Federal judge hears challenge to FDA approval of abortion drugNew: U.S. District Judge Matthew Kacsmaryk said he would rule “as soon as possible” on the request for a preliminary injunction that would move mifepristone, a common abortion-inducing drug, off the market.
Federal judge hears challenge to FDA approval of abortion drugNew: U.S. District Judge Matthew Kacsmaryk said he would rule “as soon as possible” on the request for a preliminary injunction that would move mifepristone, a common abortion-inducing drug, off the market.
Read more »
 COVID vaccine update: FDA authorizes Pfizer booster for some children under age fiveThe U.S. Food and Drug Administration (FDA) has authorized a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for children 6 months through 4 years of age.
COVID vaccine update: FDA authorizes Pfizer booster for some children under age fiveThe U.S. Food and Drug Administration (FDA) has authorized a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine, Bivalent for children 6 months through 4 years of age.
Read more »
 FDA panel to meet Thursday to discuss full approval of PaxlovidA Food and Drug Administration committee is set to meet Thursday to decide whether the regulator should grant full approval to Paxlovid, the antiviral pill...
FDA panel to meet Thursday to discuss full approval of PaxlovidA Food and Drug Administration committee is set to meet Thursday to decide whether the regulator should grant full approval to Paxlovid, the antiviral pill...
Read more »
