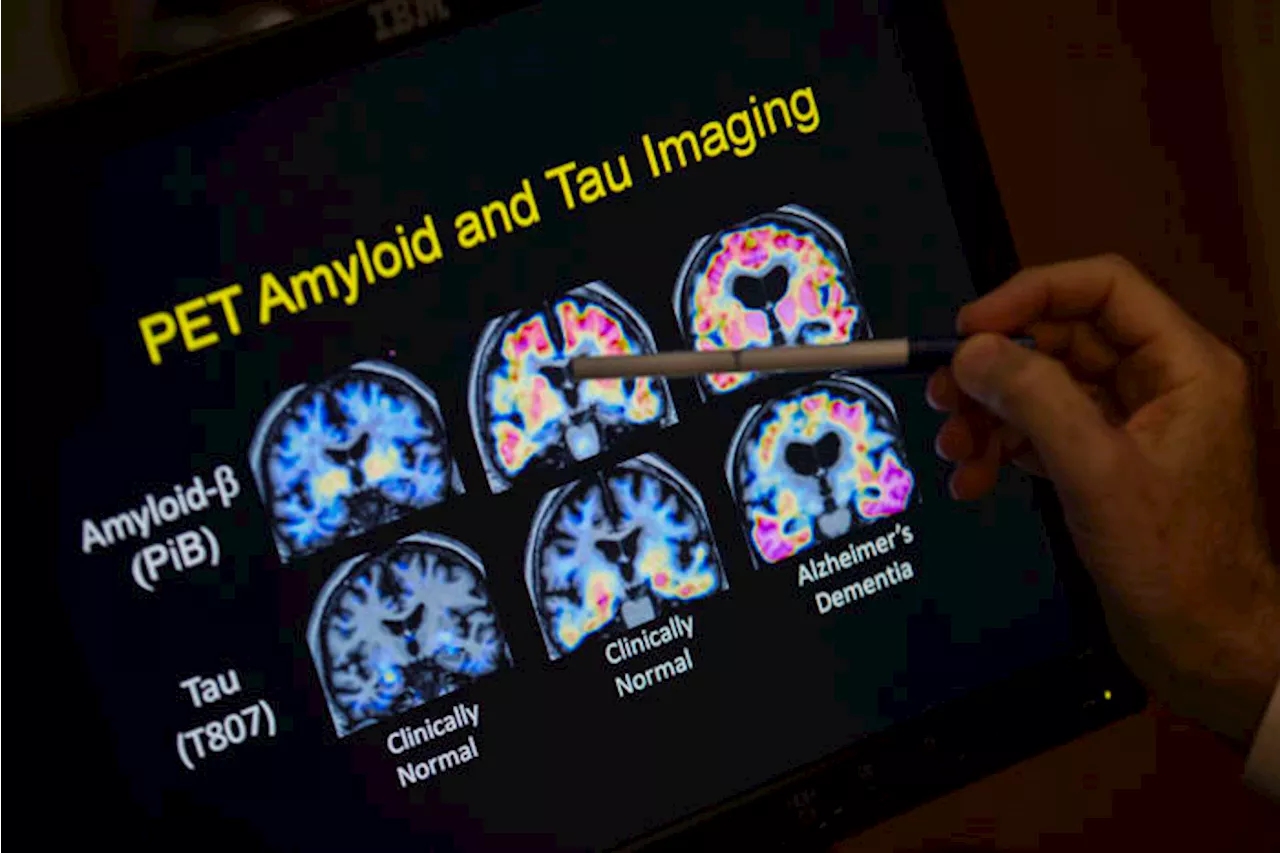
Britain’s drug regulator has authorized the Alzheimer’s drug Leqembi, saying that it’s the first medicine to show some impact in slowing progression of the neurodegenerative disease.
FILE - A doctor points to PET scan results that are part of a study on Alzheimer's disease at Georgetown University Hospital, on Tuesday, May 19, 2015, in Washington. – Britain’s drug regulator authorized the Alzheimer’s drug Leqembi on Thursday, saying it's the first medicine to show some impact in slowing the progression of the neurodegenerative disease.
The draft guidance issued by NICE will now be open for public consultation and all responses will be considered at a second meeting later this year before final advice is issued. Hilary Evans-Newton, chief executive of Alzheimers Research UK, said Leqembi represented “the beginning of a sea-change in how diseases like Alzheimer's will be treated in the future.” She said there were more than 160 trials underway testing over 125 experimental treatments for Alzheimer’s across the globe.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 European drug regulator rejects Alzheimer's treatment Leqembi from Biogen, EisaiThe committee said Leqembi's effect on delaying cognitive decline does not outweigh 'the risk of serious side effects associated with the medicine.'
European drug regulator rejects Alzheimer's treatment Leqembi from Biogen, EisaiThe committee said Leqembi's effect on delaying cognitive decline does not outweigh 'the risk of serious side effects associated with the medicine.'
Read more »
 European drug regulator rejects Alzheimer's treatment Leqembi from Biogen, EisaiThe committee said Leqembi’s effect on delaying cognitive decline does not outweigh “the risk of serious side effects associated with the…
European drug regulator rejects Alzheimer's treatment Leqembi from Biogen, EisaiThe committee said Leqembi’s effect on delaying cognitive decline does not outweigh “the risk of serious side effects associated with the…
Read more »
 European committee says Alzheimer's treatment Leqembi shouldn't get marketing approvalShares of Biogen slid Friday after a European regulatory committee said the Alzheimer’s treatment Leqembi should not receive marketing approval. The European Medicines Agency committee said concerns about the drug’s potential side effects outweigh the impact it has in slowing the fatal, mind-robbing disease.
European committee says Alzheimer's treatment Leqembi shouldn't get marketing approvalShares of Biogen slid Friday after a European regulatory committee said the Alzheimer’s treatment Leqembi should not receive marketing approval. The European Medicines Agency committee said concerns about the drug’s potential side effects outweigh the impact it has in slowing the fatal, mind-robbing disease.
Read more »
 Patients on Alzheimer's drug Leqembi see benefits over three years, Eisai study saysThe study results on Leqembi, which Eisai shares with Biogen, also found that Alzheimer’s patients who take the therapy get worse after they stop…
Patients on Alzheimer's drug Leqembi see benefits over three years, Eisai study saysThe study results on Leqembi, which Eisai shares with Biogen, also found that Alzheimer’s patients who take the therapy get worse after they stop…
Read more »
 Patients on Alzheimer's drug Leqembi see benefits over three years, Eisai study saysThe study results on Leqembi, which Eisai shares with Biogen, also found that Alzheimer’s patients who take the therapy get worse after they stop…
Patients on Alzheimer's drug Leqembi see benefits over three years, Eisai study saysThe study results on Leqembi, which Eisai shares with Biogen, also found that Alzheimer’s patients who take the therapy get worse after they stop…
Read more »
 Biogen beats expectations, hikes outlook as Alzheimer's drug Leqembi and other new products gain tractionUptake of Biogen’s breakthrough Alzheimer’s drug, Leqembi, appears to be picking up, with roughly $40 million in sales for the quarter.
Biogen beats expectations, hikes outlook as Alzheimer's drug Leqembi and other new products gain tractionUptake of Biogen’s breakthrough Alzheimer’s drug, Leqembi, appears to be picking up, with roughly $40 million in sales for the quarter.
Read more »