The first drug that slows Alzheimer's has finally received FDA approval
Besides needing a medical prescription, taking the drug will require professional administration in a hospital or infusion center every two weeks. The company, though it may not be its sole responsibility, recognizes its need to boost accessibility. In a, Christopher Viehbacher, the CEO of Biogen, said the company’s main focus now is to work with Eisai to make Leqembi “accessible to eligible patients as soon as possible.
The drug’s hefty price tag of $26,500 will unfortunately make it inaccessible to most. Current rules mean that it’s unlikely to be covered by Medicare. According to the, those on Medicaid only should be able to get coverage of the FDA-approved drug in most cases. But, even if Medicaid does cover it, patients would be responsible for a 20 percent copay – or about $5,300.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Japanese pharma Eisai slides 4% despite FDA approval for Alzheimer's drugLeqembi is the first Alzheimer's antibody treatment to receive full FDA approval.
Japanese pharma Eisai slides 4% despite FDA approval for Alzheimer's drugLeqembi is the first Alzheimer's antibody treatment to receive full FDA approval.
Read more »
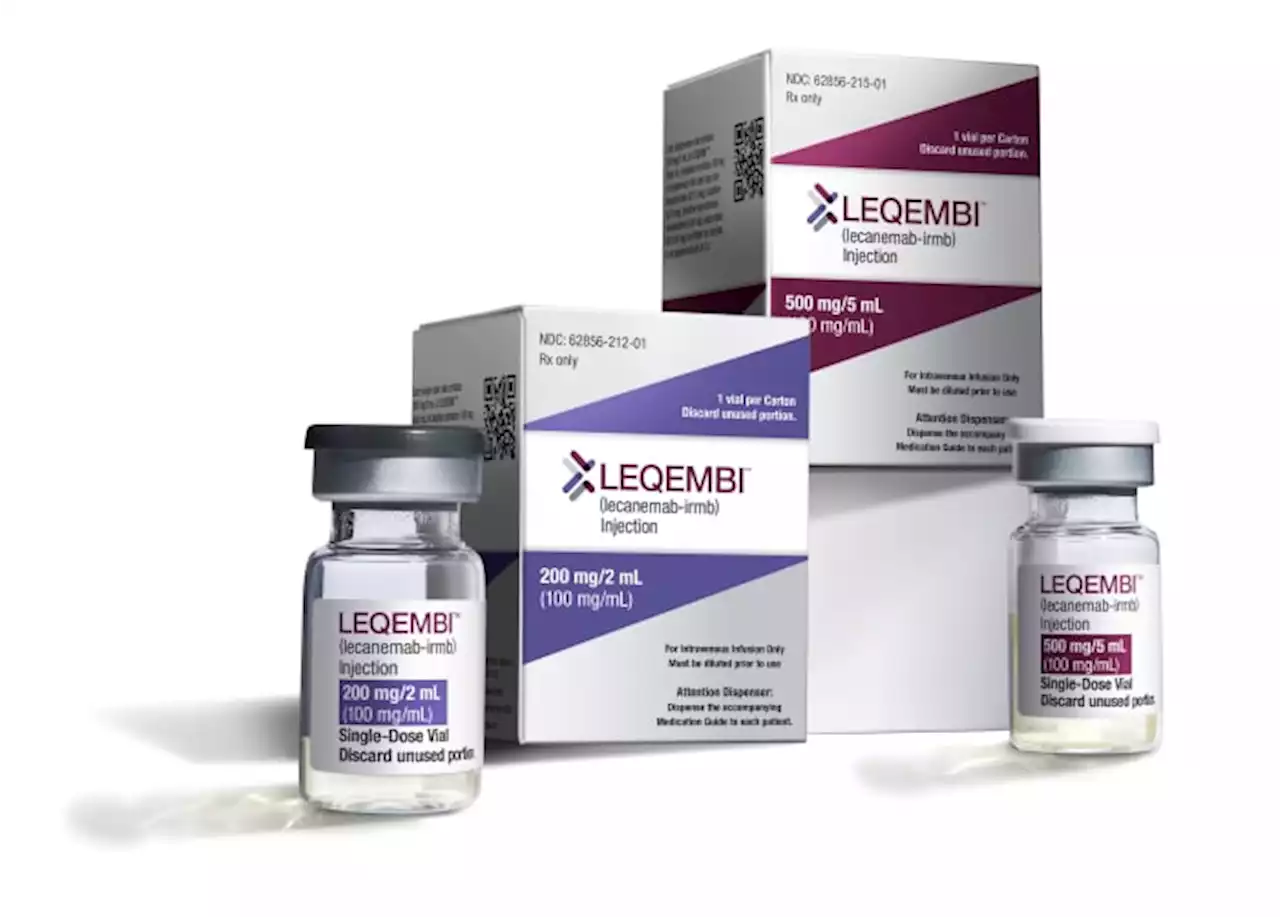 FDA to make a decision on whether to approve first Alzheimer’s drugThe FDA is set to decide whether it will fully approve the first Alzheimer’s drug to show it could slow the disease’s progression in certain patients. But the decision could also have other implications, including who would get access to it.
FDA to make a decision on whether to approve first Alzheimer’s drugThe FDA is set to decide whether it will fully approve the first Alzheimer’s drug to show it could slow the disease’s progression in certain patients. But the decision could also have other implications, including who would get access to it.
Read more »
 FDA gives full approval to first drug to clearly, but modestly, slow Alzheimer’sBreaking news: The FDA on Thursday gave full approval, for the first time, to a drug that modestly slows Alzheimer’s disease — a development that offers a degree of hope but also raises difficult questions about safety, effectiveness and cost.
FDA gives full approval to first drug to clearly, but modestly, slow Alzheimer’sBreaking news: The FDA on Thursday gave full approval, for the first time, to a drug that modestly slows Alzheimer’s disease — a development that offers a degree of hope but also raises difficult questions about safety, effectiveness and cost.
Read more »
 FDA grants first full approval for an Alzheimer’s drug in 20 yearsQuestions linger around how many patients will be able to access the drug with limited coverage from Medicare.
FDA grants first full approval for an Alzheimer’s drug in 20 yearsQuestions linger around how many patients will be able to access the drug with limited coverage from Medicare.
Read more »
 First Alzheimer's drug to slow disease, Leqembi, gets full FDA approvalLeqembi is not a cure, but it is the first drug shown to slow the progression of Alzheimer's disease. It first received an accelerated approval from the FDA earlier this year.
First Alzheimer's drug to slow disease, Leqembi, gets full FDA approvalLeqembi is not a cure, but it is the first drug shown to slow the progression of Alzheimer's disease. It first received an accelerated approval from the FDA earlier this year.
Read more »
 FDA grants full approval to Leqembi, first drug to slow progress of Alzheimer'sThe U.S. Food and Drug Administration announced full approval of lecanemab for early-stage Alzheimer's.
FDA grants full approval to Leqembi, first drug to slow progress of Alzheimer'sThe U.S. Food and Drug Administration announced full approval of lecanemab for early-stage Alzheimer's.
Read more »
