Federal health officials issued the alert for six different brands of products after finding bacterial contamination at a manufacturing facility.
The FDA warned consumers to stop using eyedrop products from six different brands on Wednesday after agency investigators found bacteria contamination at a manufacturing site.The FDA warned consumers to stop using eyedrop products from six different brands on Wednesday after agency investigators found bacteria contamination at a manufacturing site.U.S.
The federal regulatory agency said it recommended the manufacturers to recall of the subject products on Wednesday, after FDA investigators found bacterial contamination in critical drug production areas of a manufacturing facility. CVS, Rite Aid and Target are removing the products in store and online, according to the FDA. Products branded as Leader, Rugby and Velocity may still be available but should not be purchased, said the agency. None of the products have caused adverse effects in consumers yet, they added.
It's the FDA's latest statements in a series of warnings against using eyedrop products linked to potential contamination. Two months ago, theto stop using two eyedrop products due to bacterial and fungal contamination. At the time, the drug-resistant bacteria Pseudomanas aeruginosa, Mycobacterium, Mycolicibacterium and Methylorubrum was found in LightEyez MSM Eye Drops Eye Repair product; Dr. Berne's MSM Drops 5% Solution was contaminated with Exophiala fungi..
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
FDA Warns Against Probiotics for Preemie BabiesFederal regulators have sent warning letters to two companies for illegally selling probiotic products for use in preterm infants. The U.S. Food and Drug Administration also sent a letter to health care providers warning of the risks. Probiotic products contain live organisms...
Read more »
 FDA warns about giving probiotics to premature babies in wake of infant deathThe FDA is warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products.
FDA warns about giving probiotics to premature babies in wake of infant deathThe FDA is warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products.
Read more »
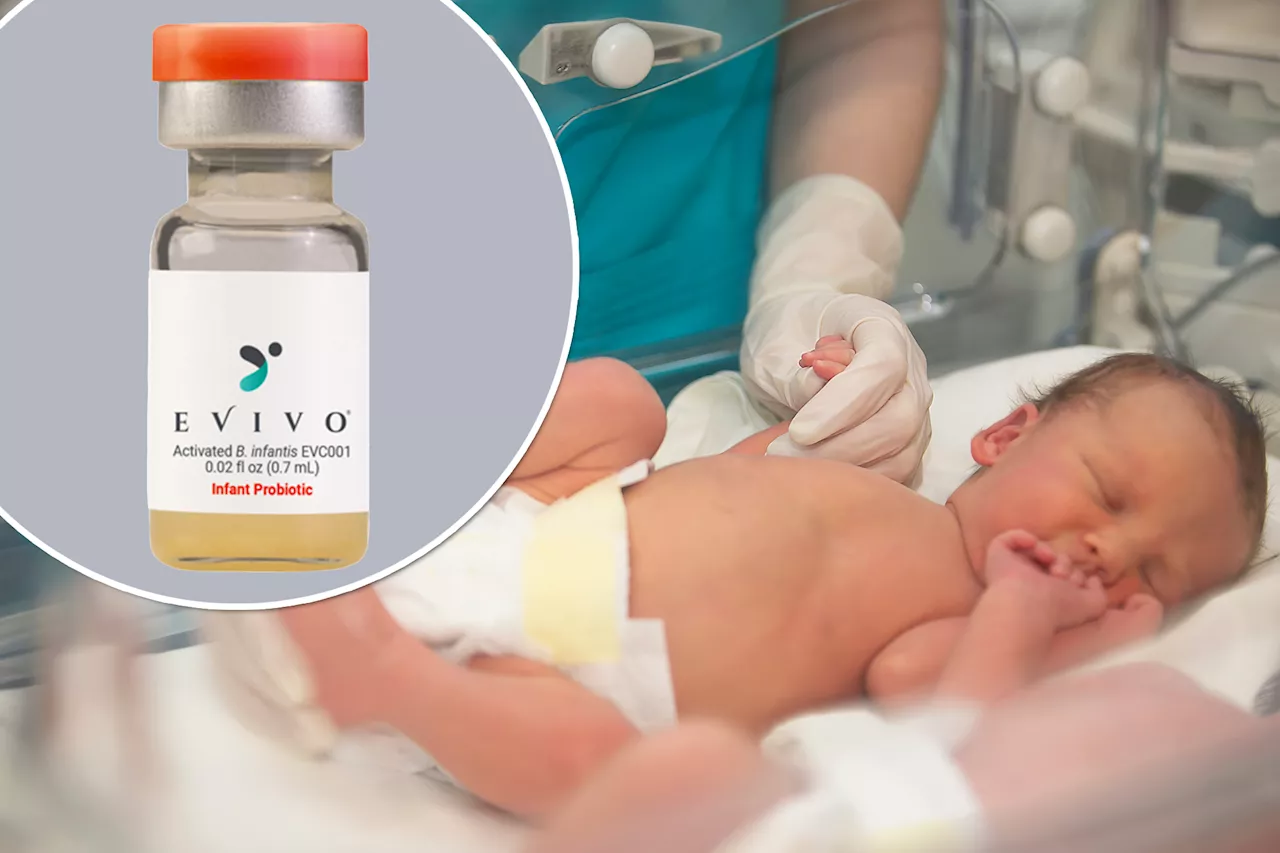 FDA issues warning about probiotic use in infants linked to death, illnesses this yearCertain probiotics, the agency noted, have been linked to one infant death this year and over two dozen “adverse events” since 2018.
FDA issues warning about probiotic use in infants linked to death, illnesses this yearCertain probiotics, the agency noted, have been linked to one infant death this year and over two dozen “adverse events” since 2018.
Read more »
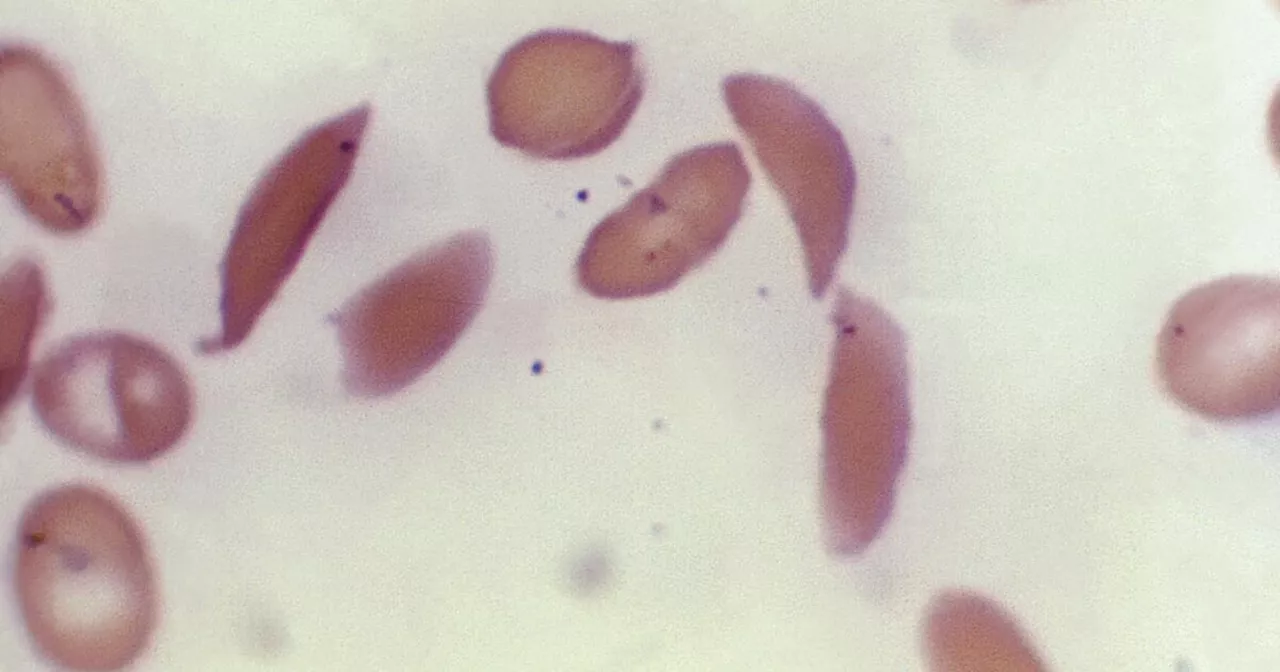 FDA revisará terapia genética para trastorno de los glóbulos rojosLa única cura para la dolorosa enfermedad de células falciformes hoy en día es un trasplante de médula ósea.
FDA revisará terapia genética para trastorno de los glóbulos rojosLa única cura para la dolorosa enfermedad de células falciformes hoy en día es un trasplante de médula ósea.
Read more »
FDA Approves Drug to Treat Ulcerative ColitisEli Lilly and Co said on Thursday that the U.S. health regulator had approved its drug for treating adults with moderate-to-severe active ulcerative colitis, a type of chronic inflammatory bowel disease. The drug, which will be available in the United States in coming weeks...
Read more »
 After infant’s death, FDA sends new warning about probiotics for premature babies in the hospitalThe US Food and Drug Administration is sharing a new warning about the risks of probiotics for hospitalized preterm infants. The products have been linked with over two dozen reported adverse events since 2018 and one death in 2023, the agency said.
After infant’s death, FDA sends new warning about probiotics for premature babies in the hospitalThe US Food and Drug Administration is sharing a new warning about the risks of probiotics for hospitalized preterm infants. The products have been linked with over two dozen reported adverse events since 2018 and one death in 2023, the agency said.
Read more »
