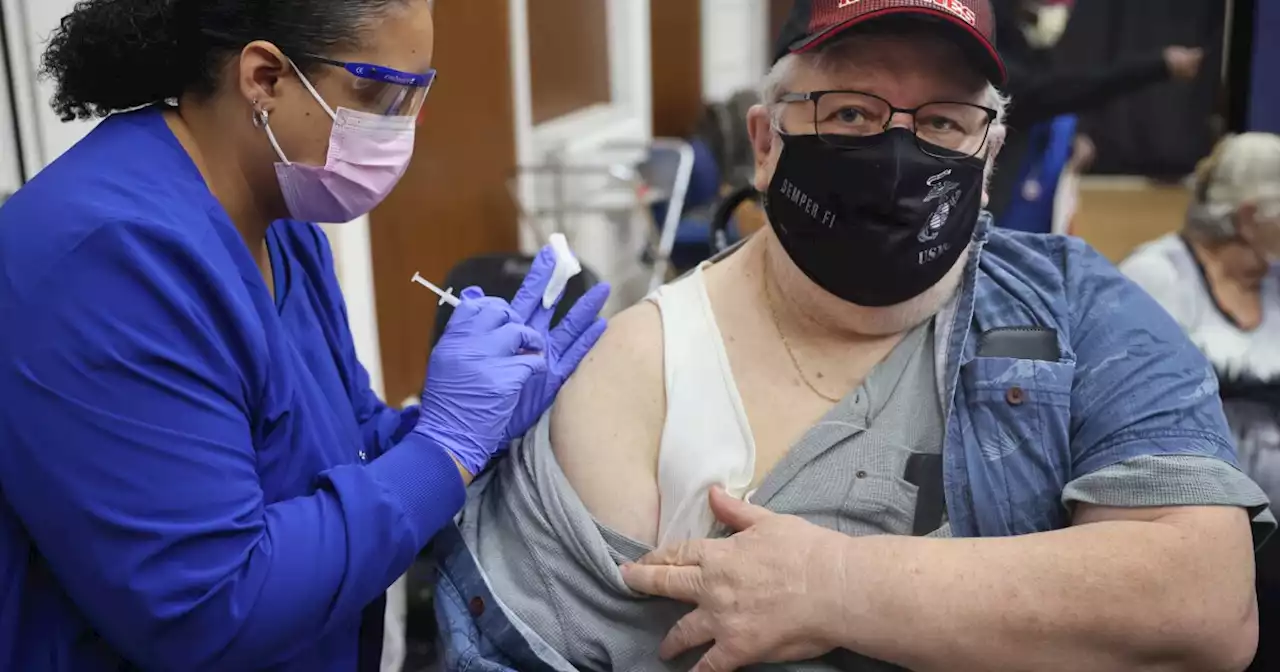
The FDA appears poised to make available the COVID-19 vaccines that target omicron as a second booster for people with weak immune systems and those ages 65 and older.
have done, and what some vaccine specialists have been urging — especially with the ample of supply of vaccine available.
The main concern is the protection people got from their last shot has been fading, not just against getting infected but also possibly against getting seriously ill. So Hotez says people as young as 50 should be able to get a second bivalent booster if they want one. But others scientists aren't so sure. They say there just isn't any good evidence showing protection against serious illness has faded significantly or that getting another shot would help that much.
"The concern is that if we continue to give boosters against a virus that's not circulating when we do see the next variant you may not develop a vigorous immune response to that new viral variant," Poland says., and so the demand for another right now would probably be even lower.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
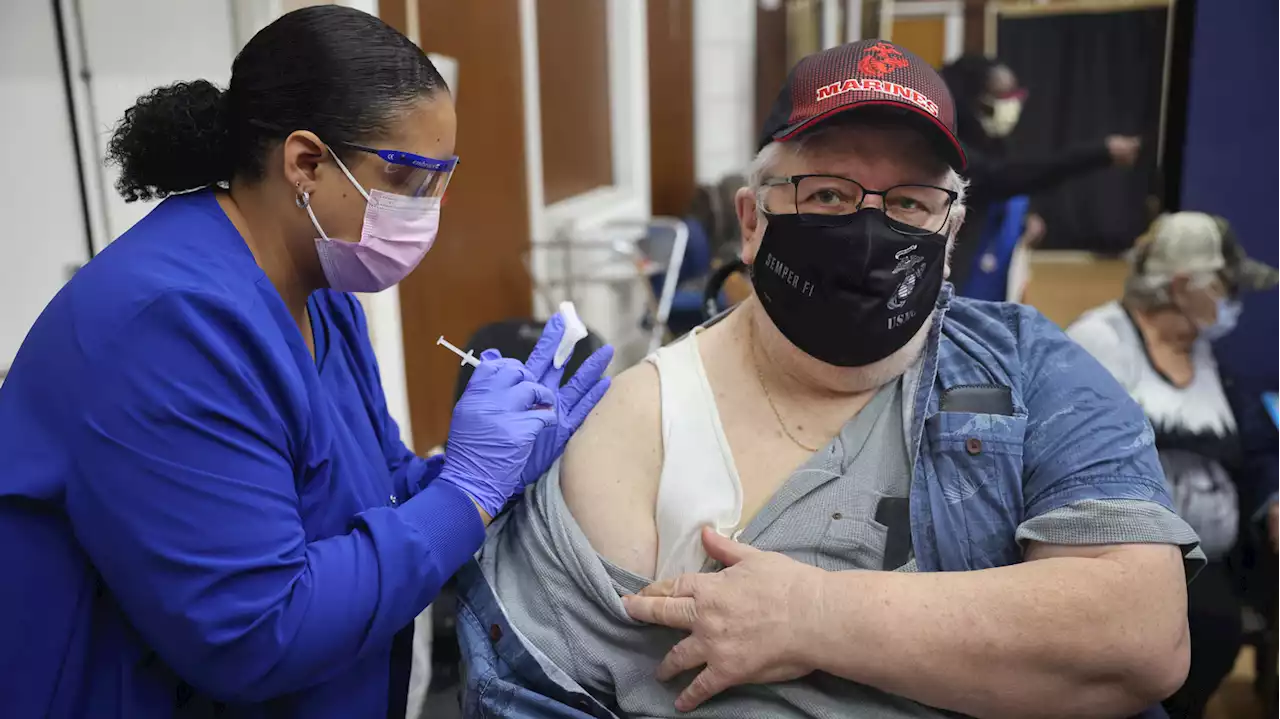 The FDA may soon authorize a spring round of COVID-19 boosters for some peopleThe Food and Drug Administration appears poised to make available the COVID-19 vaccines that target omicron as a second booster for people with weak immune systems and those ages 65 and older.
The FDA may soon authorize a spring round of COVID-19 boosters for some peopleThe Food and Drug Administration appears poised to make available the COVID-19 vaccines that target omicron as a second booster for people with weak immune systems and those ages 65 and older.
Read more »
 Melba Wilson shares her favorite recipes for meatloaf and mashed potatoesHarlem-based restaurateur joined the TODAY Food team to share two comfort food recipes.
Melba Wilson shares her favorite recipes for meatloaf and mashed potatoesHarlem-based restaurateur joined the TODAY Food team to share two comfort food recipes.
Read more »
 U.S. baby formula supply is still vulnerable, former FDA official tells lawmakersA former FDA official underscored dysfunction within the FDA that he believes exacerbated last year's nationwide infant formula shortage.
U.S. baby formula supply is still vulnerable, former FDA official tells lawmakersA former FDA official underscored dysfunction within the FDA that he believes exacerbated last year's nationwide infant formula shortage.
Read more »
 FDA approves over-the-counter Narcan to reduce drug overdosesBREAKING: FDA approves the overdose reversal drug Narcan for over-the-counter use amid the ongoing opioid epidemic.
FDA approves over-the-counter Narcan to reduce drug overdosesBREAKING: FDA approves the overdose reversal drug Narcan for over-the-counter use amid the ongoing opioid epidemic.
Read more »
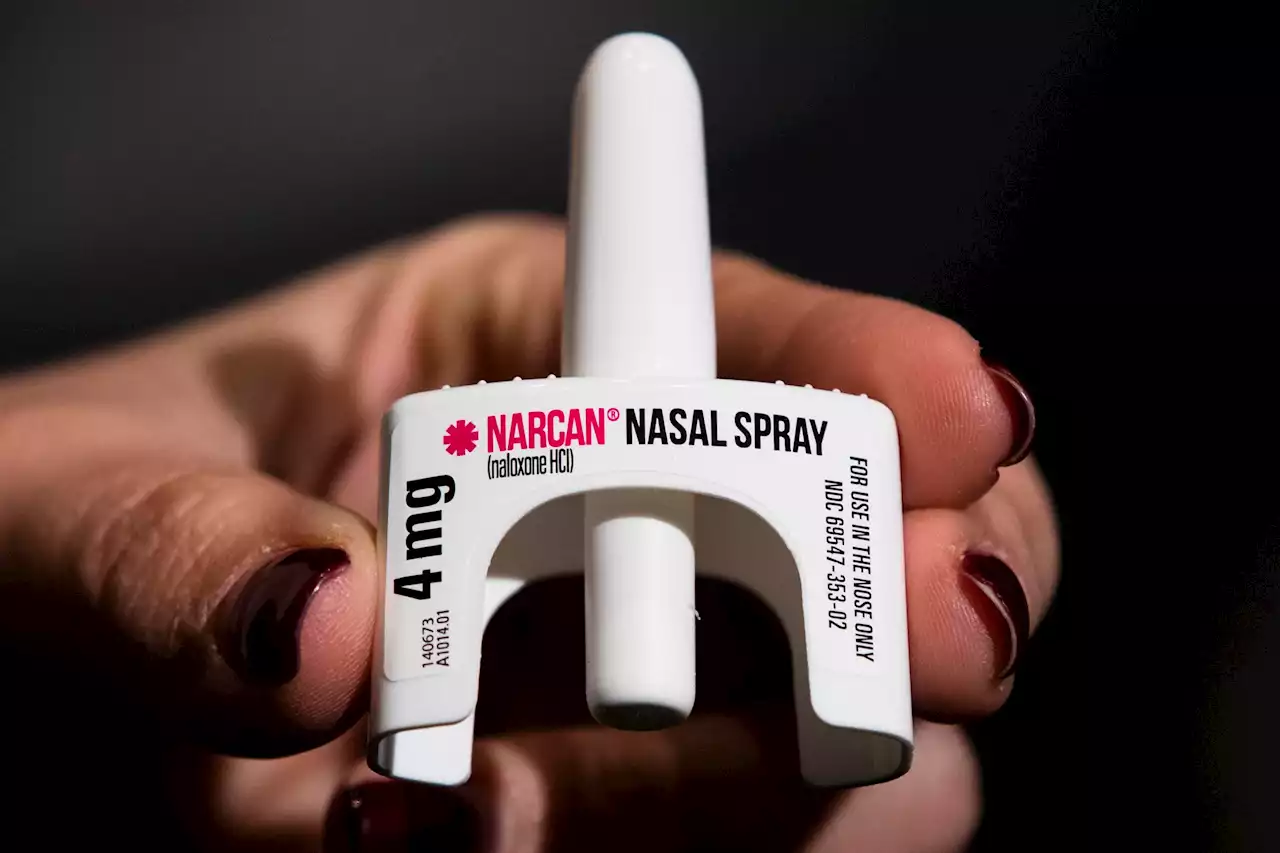 FDA Approves Narcan—An Opioid Antidote—For Over-The-Counter Use Amid Overdose EpidemicManufacturers will determine the timeline and price of the drug, the FDA said.
FDA Approves Narcan—An Opioid Antidote—For Over-The-Counter Use Amid Overdose EpidemicManufacturers will determine the timeline and price of the drug, the FDA said.
Read more »
 FDA proposes overhaul of fast-track process for cancer medsThe Food and Drug Administration is eyeing policy changes that could make drugmakers conduct more stringent trials to win fast-track approvals of cancer drugs.
FDA proposes overhaul of fast-track process for cancer medsThe Food and Drug Administration is eyeing policy changes that could make drugmakers conduct more stringent trials to win fast-track approvals of cancer drugs.
Read more »