Among other changes, the FDA also reduced the recommended time between Pfizer booster doses and first shots from six months to five.
Centers for Disease Control and PreventionThe Food and Drug Administration announced on Monday three updated recommendations regarding the Pfizer-BioNTech COVID-19 vaccine, including expanded emergency use of the Pfizer booster shot authorized for children aged 12 to 15. It’s the first booster in the US to be approved for this age group.
A booster shot is still recommended for all adults who received their second Moderna shot at least six months ago, or one Johnson & Johnson shot at least two months ago.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
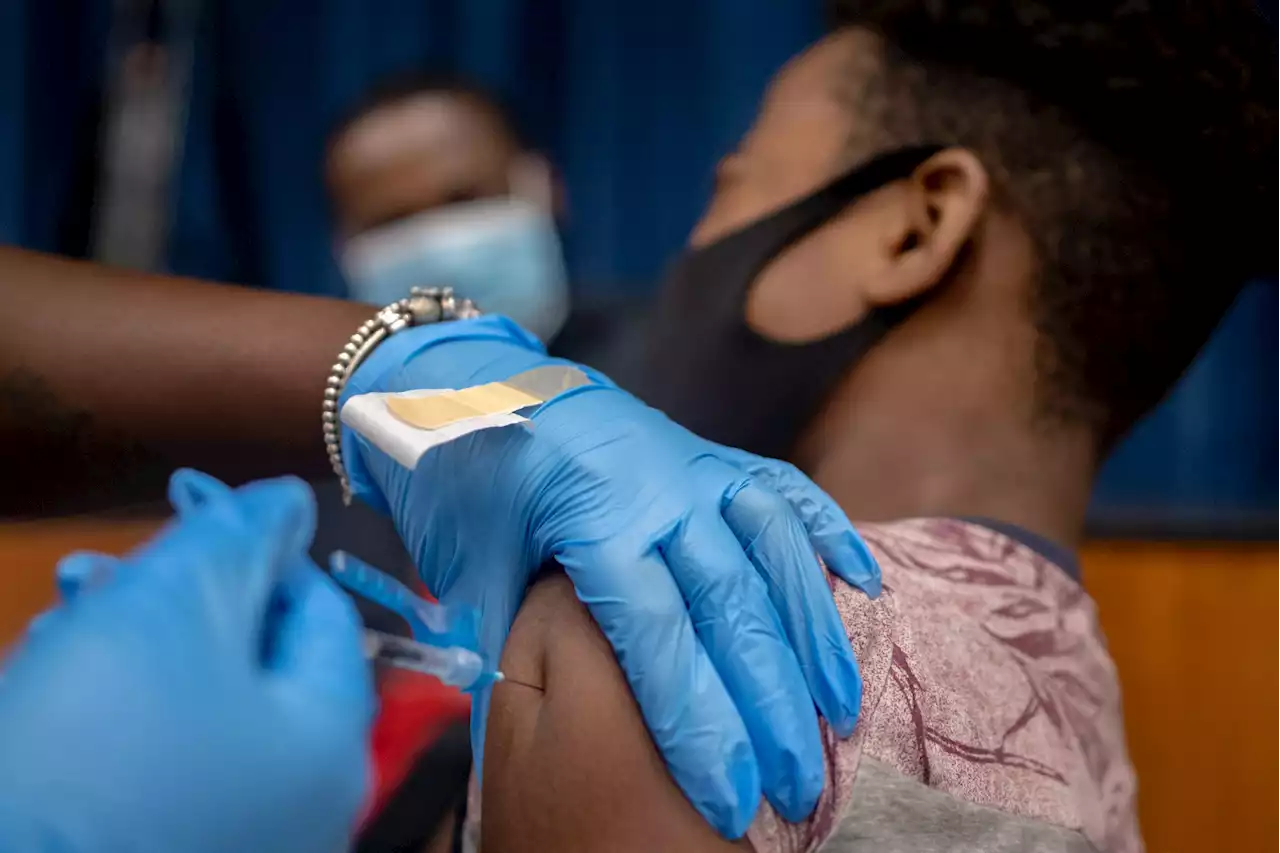 FDA authorizes Pfizer Covid-19 vaccine boosters for 12- to 15-year-oldsThe US kicked off 2022 amid a massive Covid-19 case spike — driven by the highly contagious Omicron variant — that some experts warn will be different than any other time in the pandemic.
FDA authorizes Pfizer Covid-19 vaccine boosters for 12- to 15-year-oldsThe US kicked off 2022 amid a massive Covid-19 case spike — driven by the highly contagious Omicron variant — that some experts warn will be different than any other time in the pandemic.
Read more »
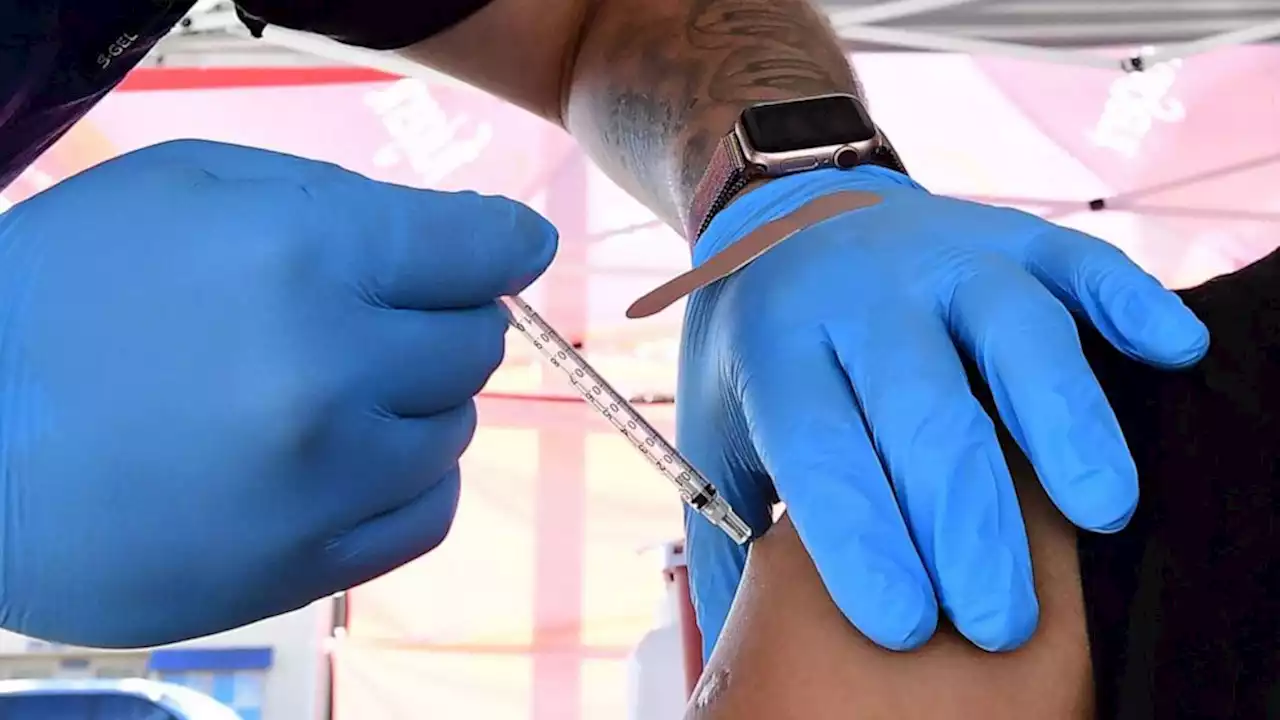 FDA authorizes Pfizer COVID-19 boosters for 12- to 15-year oldsThe FDA on Monday authorized Pfizer COVID-19 boosters for 12- to 15-year olds amid the omicron surge as children head back to school.
FDA authorizes Pfizer COVID-19 boosters for 12- to 15-year oldsThe FDA on Monday authorized Pfizer COVID-19 boosters for 12- to 15-year olds amid the omicron surge as children head back to school.
Read more »
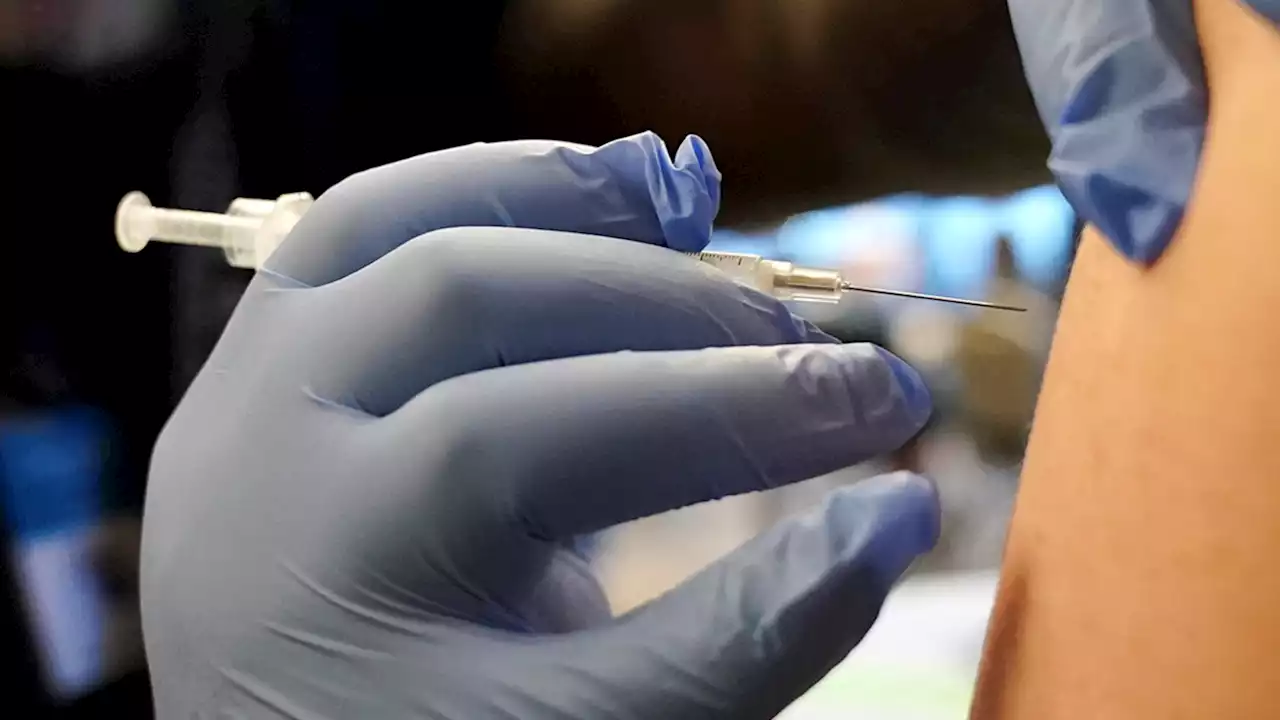 FDA expands Pfizer COVID-19 boosters for 12- to 15-year-olds as omicron surgesThe U.S. is expanding COVID-19 boosters as it confronts the omicron surge, with the FDA allowing extra Pfizer shots for children as young as 12.
FDA expands Pfizer COVID-19 boosters for 12- to 15-year-olds as omicron surgesThe U.S. is expanding COVID-19 boosters as it confronts the omicron surge, with the FDA allowing extra Pfizer shots for children as young as 12.
Read more »
 FDA Authorizes Pfizer Boosters for Kids Ages 12 to 15The U.S. is expanding COVID-19 boosters as it confronts a surge of infections nationwide, with the Food and Drug Administration allowing extra Pfizer shots for children as young as 12.
FDA Authorizes Pfizer Boosters for Kids Ages 12 to 15The U.S. is expanding COVID-19 boosters as it confronts a surge of infections nationwide, with the Food and Drug Administration allowing extra Pfizer shots for children as young as 12.
Read more »
