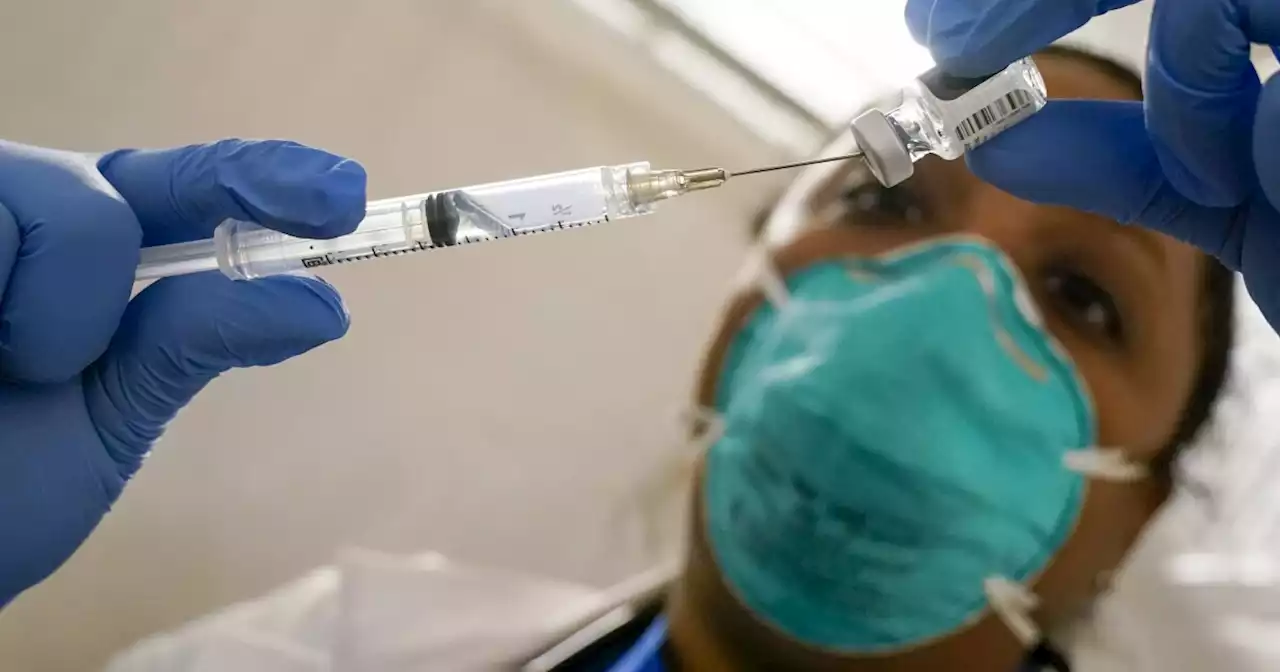
The FDA will likely authorize a secondary booster dose for adults 50 years and older this week.
Once approved by the FDA, it will be up to the CDC to follow with a “permissive recommendation," CNN reports.
That means the second booster dose won’t necessarily be recommended, but if people wish to receive it, they can.There is evidence that shows protection from three shots is waning and that a fourth shot could help boost protection – including data from Israel involving people over the age of 60.However, critics say there isn’t enough evidence to show the fourth shot is actually needed, or how long protection from it would last.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
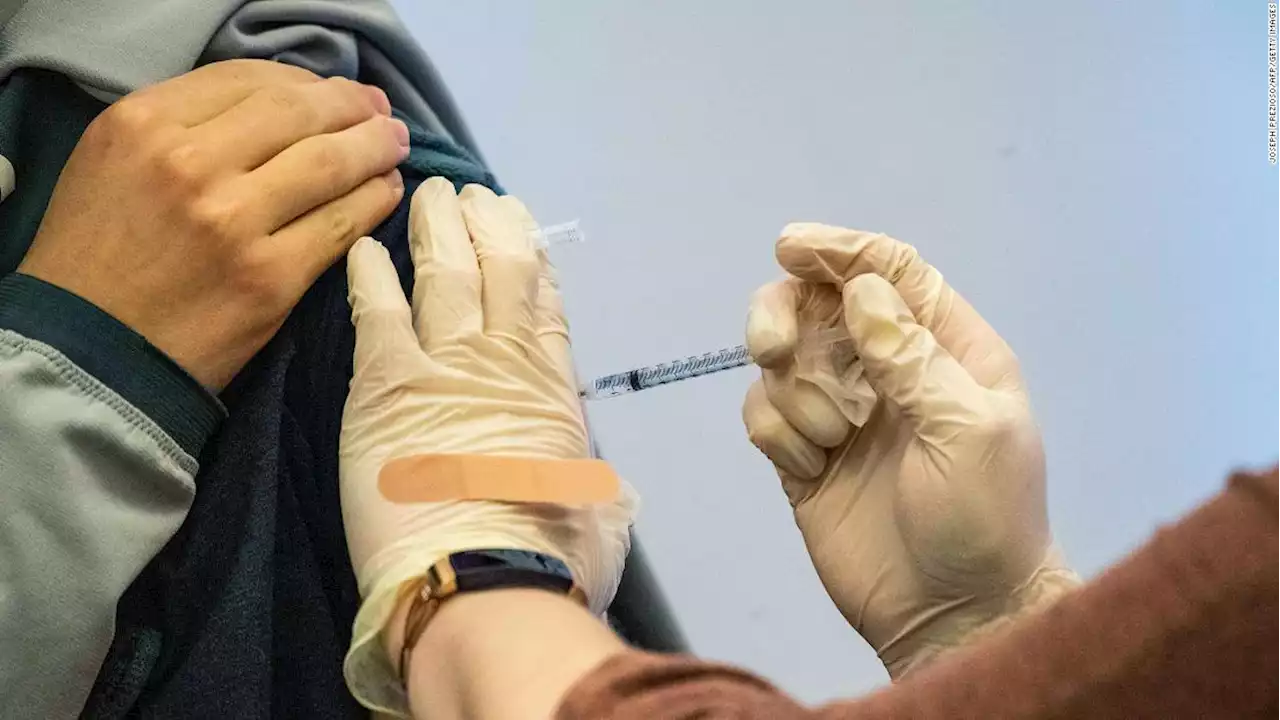 FDA expected to OK additional Covid-19 booster shots for adults over 50 next weekTwo sources familiar with the government's plans said the US Food and Drug Administration is planning authorize a fourth dose of the Pfizer and Moderna mRNA vaccines for adults over age 50 next week.
FDA expected to OK additional Covid-19 booster shots for adults over 50 next weekTwo sources familiar with the government's plans said the US Food and Drug Administration is planning authorize a fourth dose of the Pfizer and Moderna mRNA vaccines for adults over age 50 next week.
Read more »
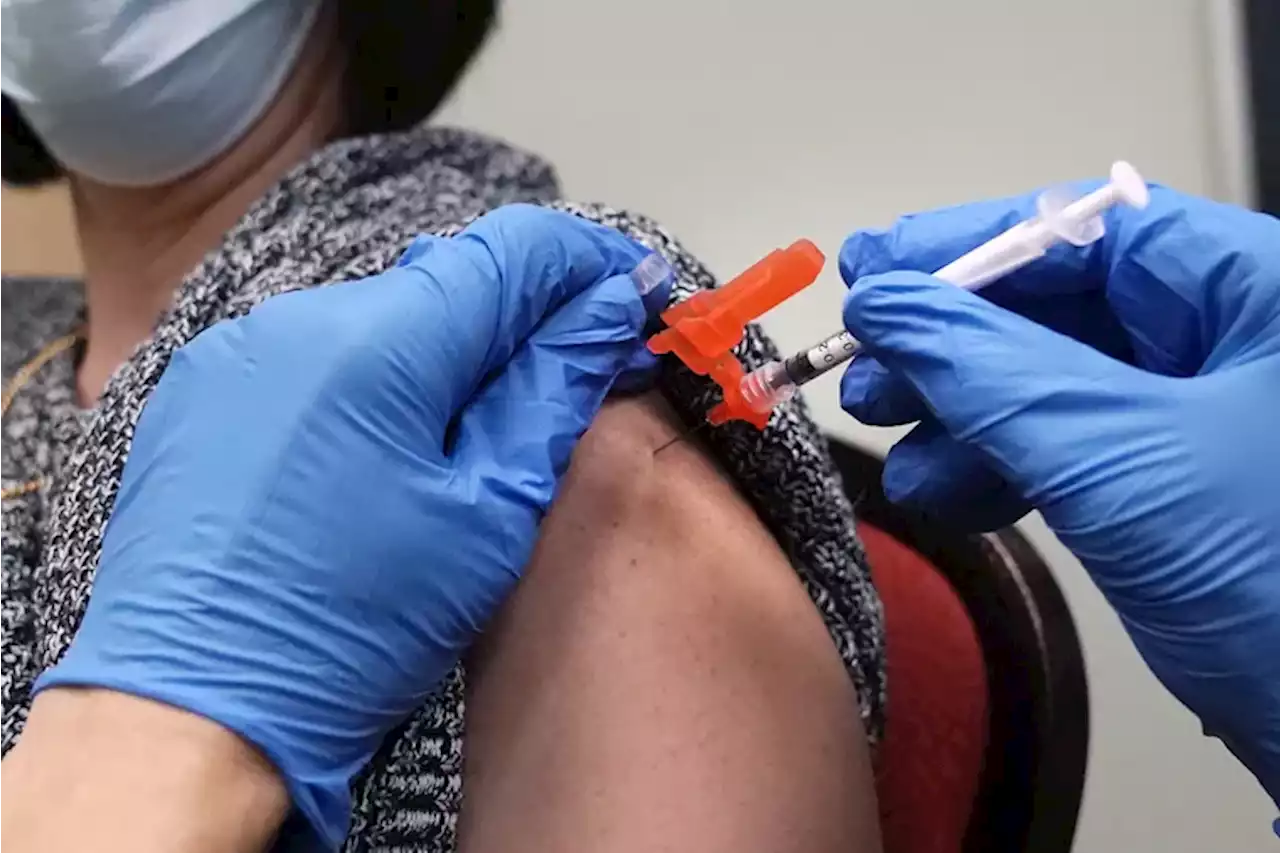 The FDA is expected to clear an additional COVID-19 booster shotThe FDA is poised to clear a fourth dose of the mRNA coronavirus vaccine for adults age 50 and older, looking to shore up protections for more vulnerable groups, a person familiar with the matter said.
The FDA is expected to clear an additional COVID-19 booster shotThe FDA is poised to clear a fourth dose of the mRNA coronavirus vaccine for adults age 50 and older, looking to shore up protections for more vulnerable groups, a person familiar with the matter said.
Read more »
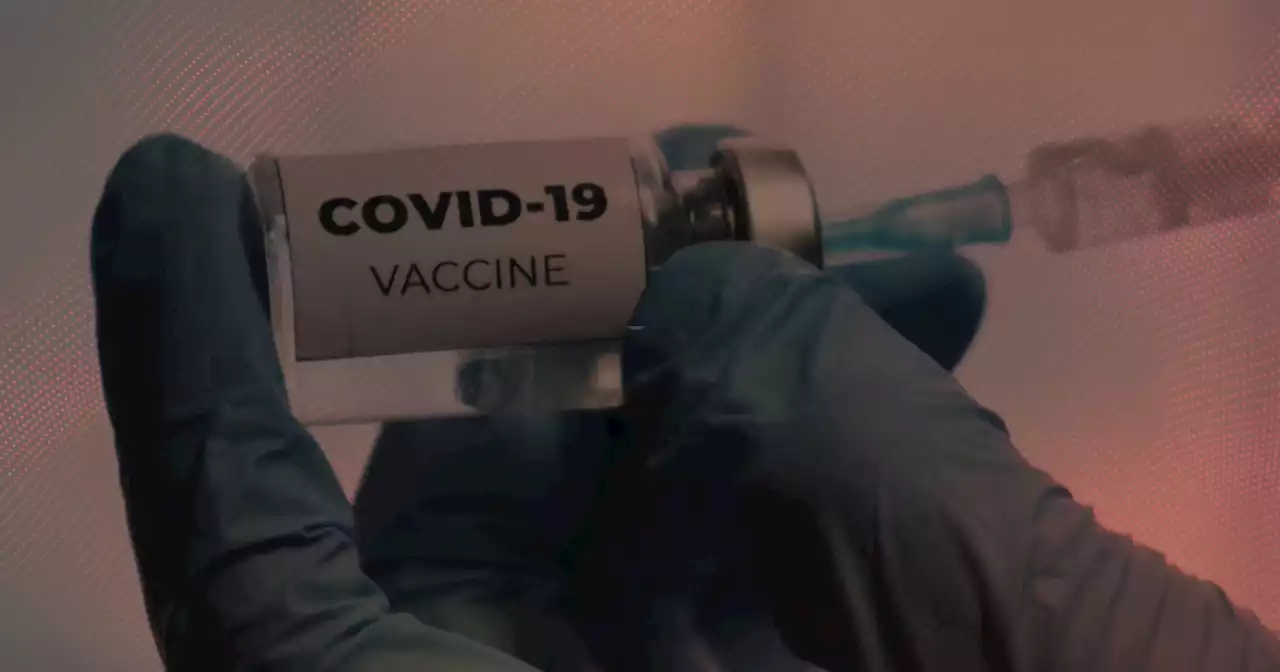 ABC News: FDA could authorize second booster shots for Americans over 50 this weekTwo officials familiar with the matter tell ABC News the FDA could authorize second booster shots for Americans over 50 years old as soon as Tuesday, but the details are still under discussion.
ABC News: FDA could authorize second booster shots for Americans over 50 this weekTwo officials familiar with the matter tell ABC News the FDA could authorize second booster shots for Americans over 50 years old as soon as Tuesday, but the details are still under discussion.
Read more »
 The FDA is expected to authorize 2nd boosters for people 50 and upPeople aged 50 and over could soon be eligible for a second Pfizer-BioNTech or Moderna COVID vaccine booster. The administration wants to offer the shots as immunity from the first booster is waning.
The FDA is expected to authorize 2nd boosters for people 50 and upPeople aged 50 and over could soon be eligible for a second Pfizer-BioNTech or Moderna COVID vaccine booster. The administration wants to offer the shots as immunity from the first booster is waning.
Read more »