A team has found a way to make the bacterial enzyme histidine kinase water-soluble, which could make it possible to rapidly screen potential antibiotics that might interfere with its functions.
A bacterial enzyme called histidine kinase is a promising target for new classes of antibiotics. However, it has been difficult to develop drugs that target this enzyme, because it is a"hydrophobic" protein that loses its structure once removed from its normal location in the cell membrane.
No existing antibiotics target histidine kinase, so drugs that disrupt these functions could represent a new class of antibiotics. Such drug candidates are badly needed to combat the growing problem of antibiotic resistance. In 2018, Zhang and his colleagues devised a simple way to convert these proteins into water-soluble versions, which maintain their structure in water. Their technique is known as the QTY code, for the letters that represent the hydrophilic amino acids that become incorporated into the proteins. Leucine becomes glutamine , isoleucine and valine become threonine , and phenylalanine becomes tyrosine .
The research team chose to focus on histidine kinase in part because of its potential as an antibiotic target. Currently most antibiotics work by damaging bacterial cell walls or interfering with the synthesis of ribosomes, the cell organelles that manufacture proteins. None of them target histidine kinase, an important bacterial protein that regulates processes such as antibiotic resistance and cell-to-cell communication.
They also found that if they only replaced the buried hydrophobic amino acids in the transmembrane segment, the protein would not retain its function. The hydrophobic amino acids have to be replaced throughout the transmembrane segment, which helps the molecule maintain the structural relationships it needs to function normally.
Pharmacology HIV And AIDS Lung Cancer Molecular Biology Cell Biology Genetics Biotechnology
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
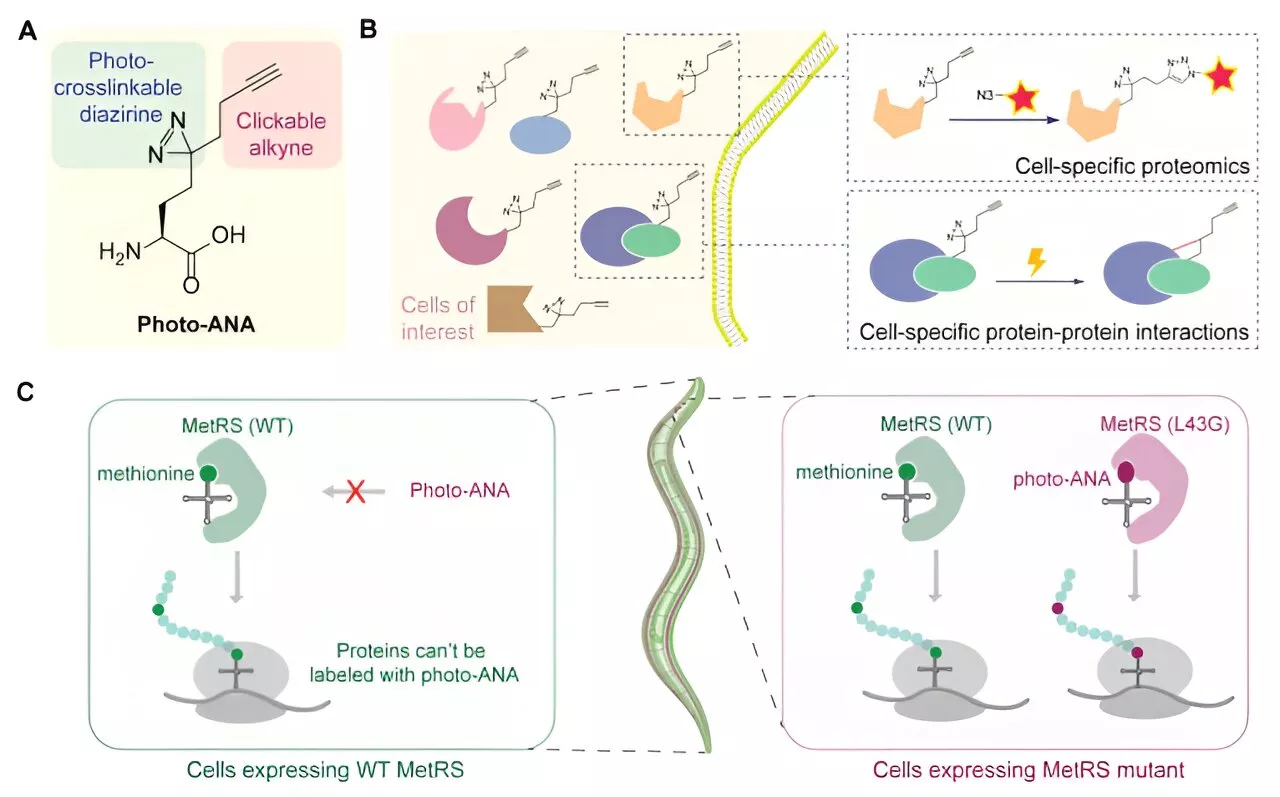 Scientists develop novel approach to interrogate tissue-specific protein–protein interactionsMulticellular organisms, like animals and plants, have complex cells with diverse functions. This complexity arises from the need for cells to produce distinct proteins that interact with each other. This interaction is crucial for cells to carry out their specific tasks and to form complex molecular machinery.
Scientists develop novel approach to interrogate tissue-specific protein–protein interactionsMulticellular organisms, like animals and plants, have complex cells with diverse functions. This complexity arises from the need for cells to produce distinct proteins that interact with each other. This interaction is crucial for cells to carry out their specific tasks and to form complex molecular machinery.
Read more »
 Novel approach to interrogate tissue-specific protein-protein interactionsMulticellular organisms, like animals and plants, have complex cells with diverse functions. This complexity arises from the need for cells to produce distinct proteins that interact with each other. This interaction is crucial for cells to carry out their specific tasks and to form complex molecular machinery.
Novel approach to interrogate tissue-specific protein-protein interactionsMulticellular organisms, like animals and plants, have complex cells with diverse functions. This complexity arises from the need for cells to produce distinct proteins that interact with each other. This interaction is crucial for cells to carry out their specific tasks and to form complex molecular machinery.
Read more »
 Study reveals insights into protein evolutionRice University's Peter Wolynes and his research team have unveiled a breakthrough in understanding how specific genetic sequences, known as pseudogenes, evolve. Their paper was published May 13 in the Proceedings of the National Academy of Sciences.
Study reveals insights into protein evolutionRice University's Peter Wolynes and his research team have unveiled a breakthrough in understanding how specific genetic sequences, known as pseudogenes, evolve. Their paper was published May 13 in the Proceedings of the National Academy of Sciences.
Read more »
 Understanding plant breathing: Study identifies the key protein interplay behind rhythmic stomatal movementsIn a study published in Nature Communications, researchers have deciphered the molecular mechanism that regulates the rhythmic movements of stomata throughout the day.
Understanding plant breathing: Study identifies the key protein interplay behind rhythmic stomatal movementsIn a study published in Nature Communications, researchers have deciphered the molecular mechanism that regulates the rhythmic movements of stomata throughout the day.
Read more »
 New study reveals key protein that could help prevent excessive bone loss in osteoporosisOsteoporosis is characterized by the weakening of bones, making them fragile and prone to breakage. Excessive activity of 'osteoclasts' or bone-absorbing cells leads to bone loss. Targeting osteoclast differentiation is therefore, a potential therapeutic strategy.
New study reveals key protein that could help prevent excessive bone loss in osteoporosisOsteoporosis is characterized by the weakening of bones, making them fragile and prone to breakage. Excessive activity of 'osteoclasts' or bone-absorbing cells leads to bone loss. Targeting osteoclast differentiation is therefore, a potential therapeutic strategy.
Read more »
 Weight Loss Wonders: New Study Uncovers Surprising Benefits of the Protein KallistatinScience, Space and Technology News 2024
Weight Loss Wonders: New Study Uncovers Surprising Benefits of the Protein KallistatinScience, Space and Technology News 2024
Read more »
