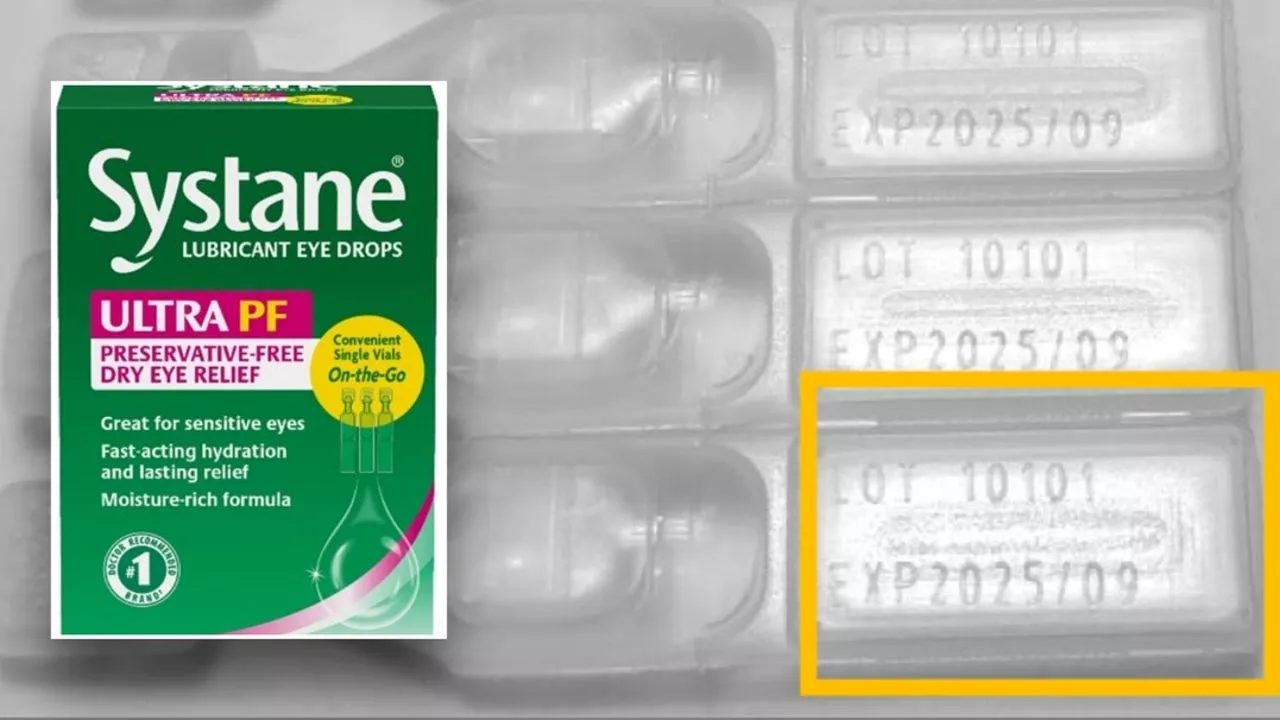
The Food and Drug Administration (FDA) has announced a nationwide recall of a single lot of Systane Lubricant Eye Drops Ultra SPF due to potential fungal contamination.
A popular brand of eye drops is being recalled nationwide due to a possible contamination, which may cause vision damage, according to the Food and Drug Administration ( FDA ).On Monday, the FDA announced that Alcon Laboratories, based out of Texas, was voluntarily recalling a single lot of 'Systane Lubricant Eye Drops Ultra SPF, Single Vials On-the-Go' as the products may be contaminated with fungus.
The company reported a consumer complaint that a 'foreign material' was found inside a sealed single-use vial and determined the material to be 'fungal in nature.'Fungal contamination in eye products are known to potentially cause eye infections, the FDA said. If an infection occurs, the FDA said it may be vision-threatening, and in very rare cases potentially life-threatening in immunocompromised patients. To date, the FDA said that Alcon Laboratories has not received any reports of adverse events related to this recall.The FDA says the affected product includes the Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, 25 count and that it is limited to lot number 10101, expiration date 2025/09. The product can be identified by the green and pink carton design, the presence of 'Systane' and 'ULTRA PF' brand names on the front of the carton, and the '25 vials' package size, the FDA explained in a press release.The lot of the potentially affected eye drops were also distributed nationwide to retail and internet outlets. Consumers who have the recalled eye drops are urged to stop using them immediately and return them to the place of purchase for a replacement or refund, the FDA said. Distributors or retailers who have the recalled eye drops are also being urged to discard any remaining stock of the tainted produc
RECALL EYE DROPS FUNGAL CONTAMINATION FDA VISION DAMAGE
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
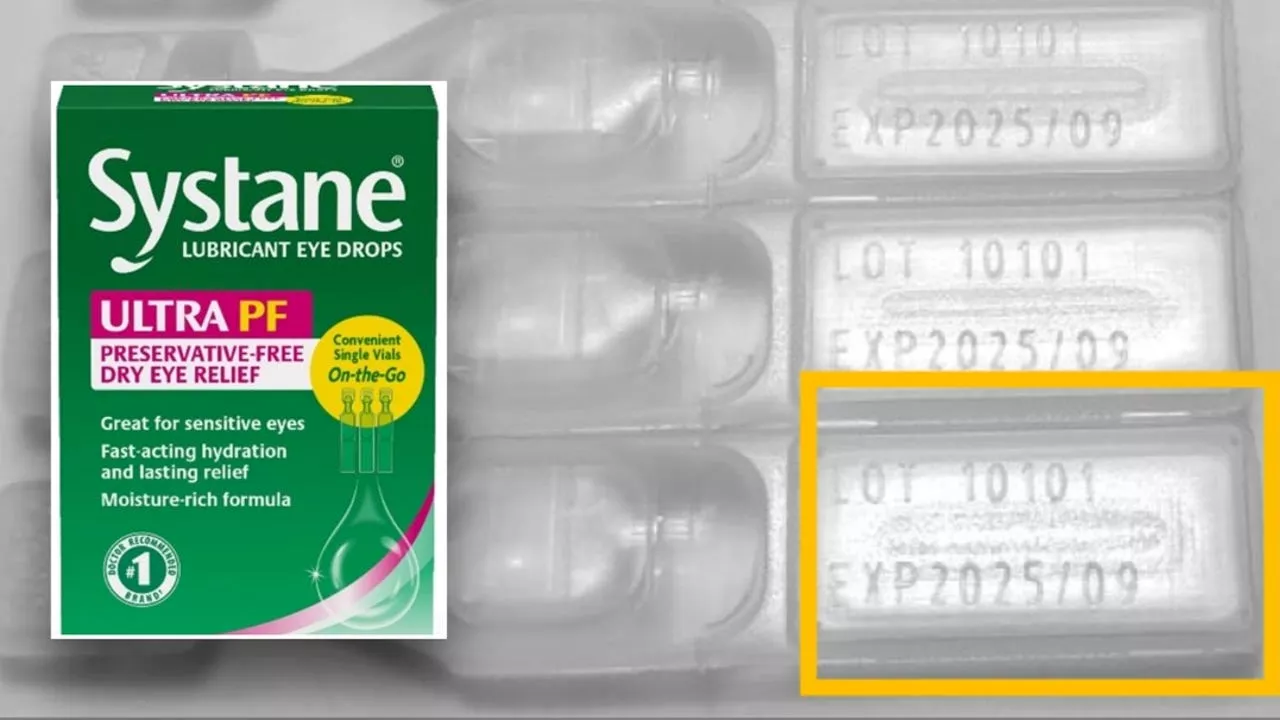 Popular Eye Drops Recalled Due to Fungus ContaminationThe FDA announced a nationwide recall of a specific lot of Systane Lubricant Eye Drops Ultra SPF due to potential fungal contamination. The contaminated product may cause eye infections, and in rare cases, vision or life-threatening complications.
Popular Eye Drops Recalled Due to Fungus ContaminationThe FDA announced a nationwide recall of a specific lot of Systane Lubricant Eye Drops Ultra SPF due to potential fungal contamination. The contaminated product may cause eye infections, and in rare cases, vision or life-threatening complications.
Read more »
 Popular Eye Drops Recalled Nationwide Due to Fungal ContaminationAlcon Laboratories is voluntarily recalling Systane Lubricant Eye Drops Ultra P-F due to a possible fungal contamination. The recall was initiated after Alcon investigated a consumer complaint of foreign material in a sealed vial. While no injuries have been reported, fungal contamination can cause eye infections. Consumers are urged to stop using the recalled eye drops and return them for a full refund.
Popular Eye Drops Recalled Nationwide Due to Fungal ContaminationAlcon Laboratories is voluntarily recalling Systane Lubricant Eye Drops Ultra P-F due to a possible fungal contamination. The recall was initiated after Alcon investigated a consumer complaint of foreign material in a sealed vial. While no injuries have been reported, fungal contamination can cause eye infections. Consumers are urged to stop using the recalled eye drops and return them for a full refund.
Read more »
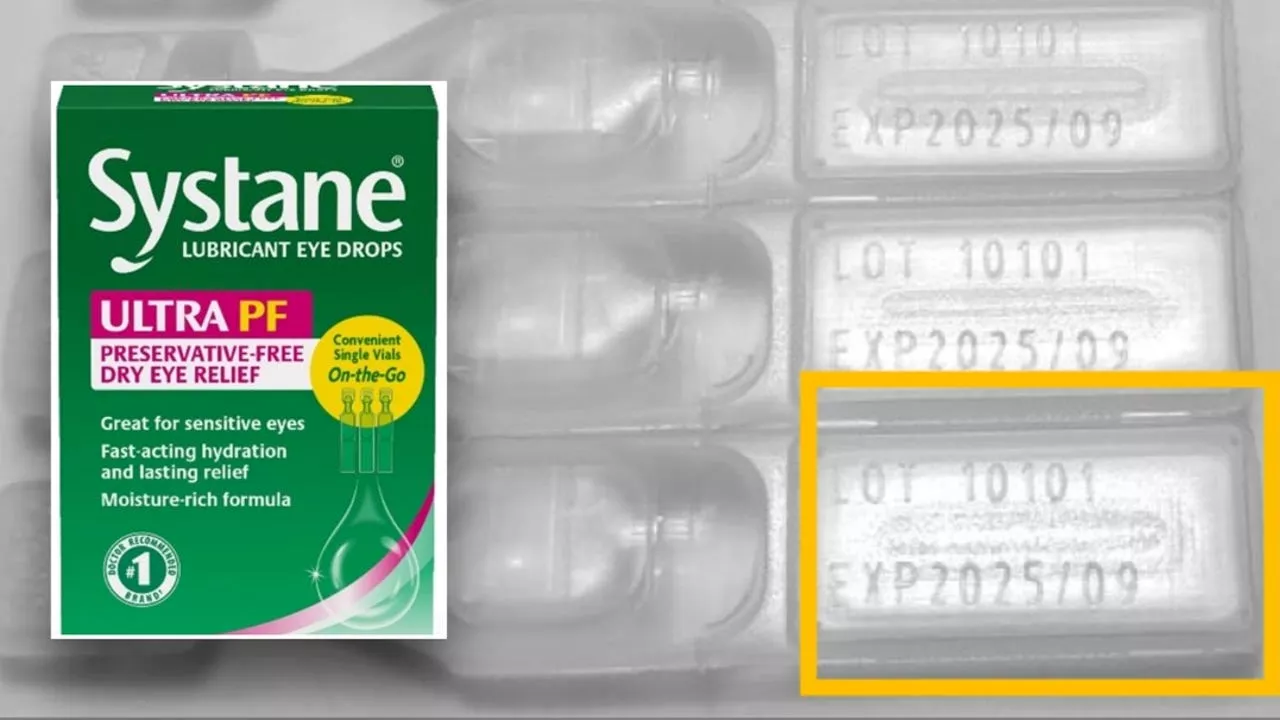 Alcon Eye Drops Recalled Due to Possible Fungus ContaminationA single lot of Systane Lubricant Eye Drops Ultra SPF has been recalled due to a possible fungal contamination, posing a risk of eye infections and vision damage.
Alcon Eye Drops Recalled Due to Possible Fungus ContaminationA single lot of Systane Lubricant Eye Drops Ultra SPF has been recalled due to a possible fungal contamination, posing a risk of eye infections and vision damage.
Read more »
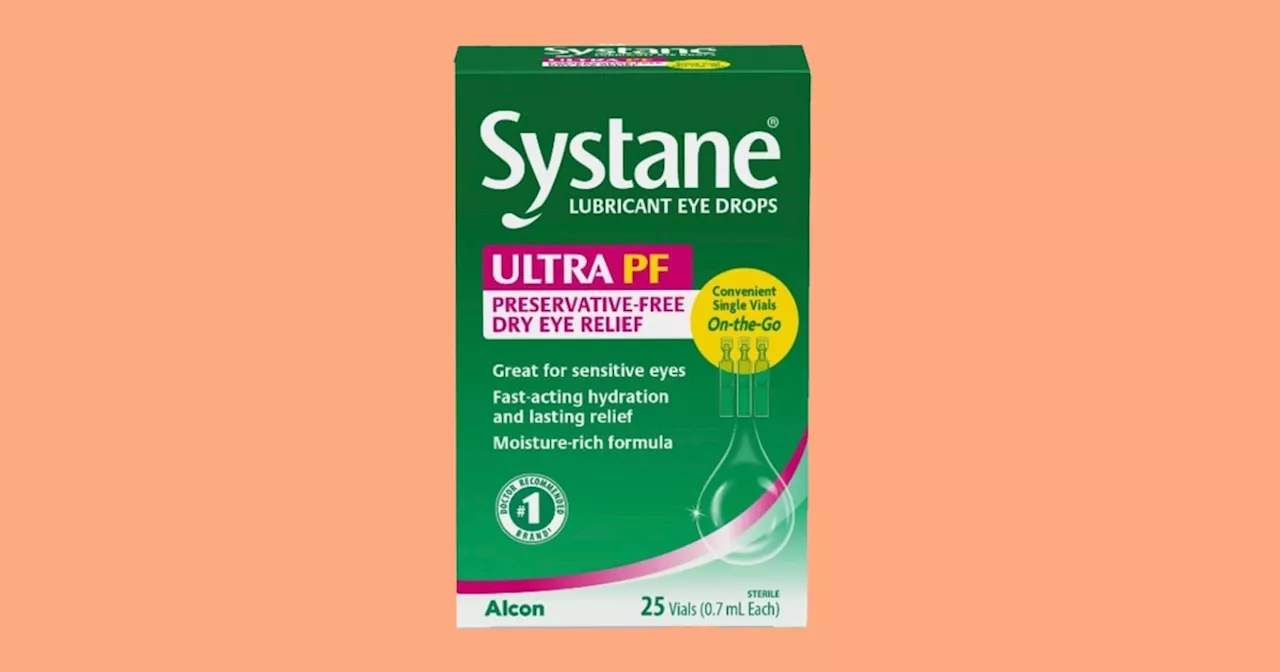 Systane eye drops recalled for possible fungal contamination, FDA saysMinyvonne Burke is a senior breaking news reporter for NBC News.
Systane eye drops recalled for possible fungal contamination, FDA saysMinyvonne Burke is a senior breaking news reporter for NBC News.
Read more »
 Sky Harbor shooting investigation; eye drops recalled over fungal concernsPolice are investigating a shooting and stabbing at Sky Harbor Airport's Terminal 4 in Phoenix; also at the airport in an unrelated case, a man was arrested after bringing firearms and getting into a fight with officers.
Sky Harbor shooting investigation; eye drops recalled over fungal concernsPolice are investigating a shooting and stabbing at Sky Harbor Airport's Terminal 4 in Phoenix; also at the airport in an unrelated case, a man was arrested after bringing firearms and getting into a fight with officers.
Read more »
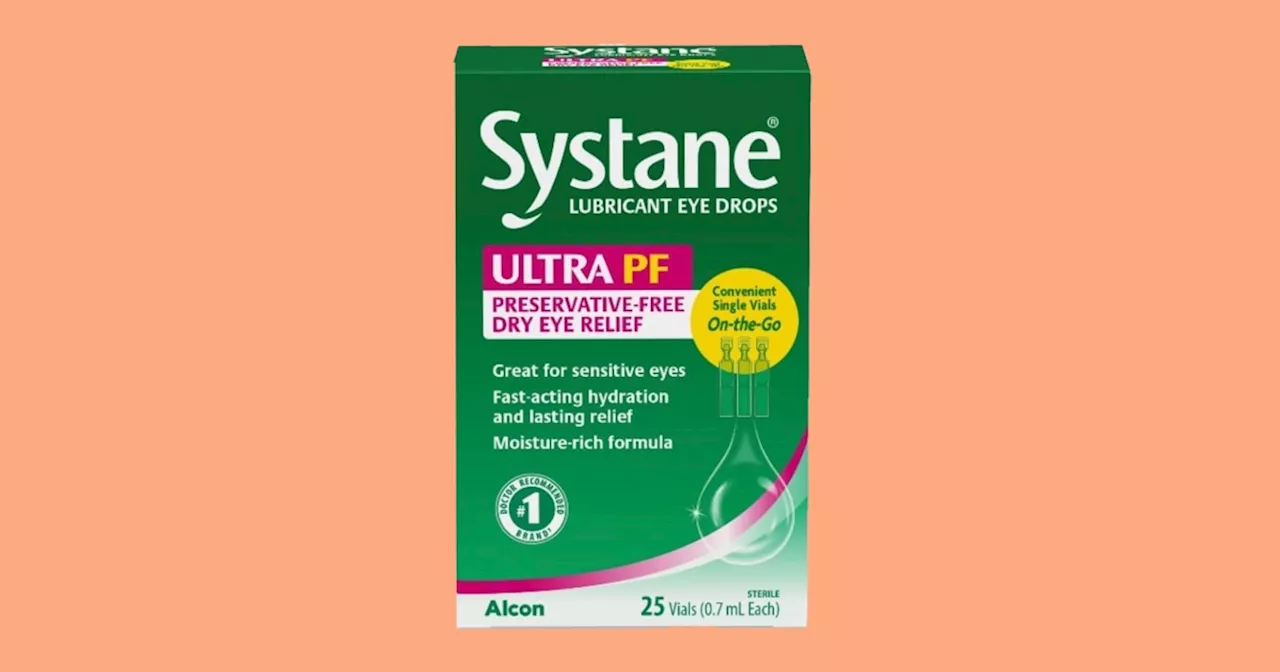 Systane Eye Drops Recalled Due to Possible Fungal ContaminationA specific lot of Systane Lubricant Eye Drops Ultra PF has been recalled following a consumer complaint about a foreign material inside a vial. The material was identified as fungal, raising concerns about potential eye infections.
Systane Eye Drops Recalled Due to Possible Fungal ContaminationA specific lot of Systane Lubricant Eye Drops Ultra PF has been recalled following a consumer complaint about a foreign material inside a vial. The material was identified as fungal, raising concerns about potential eye infections.
Read more »