A third pediatric dose of the Pfizer-BioNTech COVID-19 vaccine in children 6 months to under 5 years of age prompted a strong immune response, with a safety profile that was similar to placebo, the companies said:
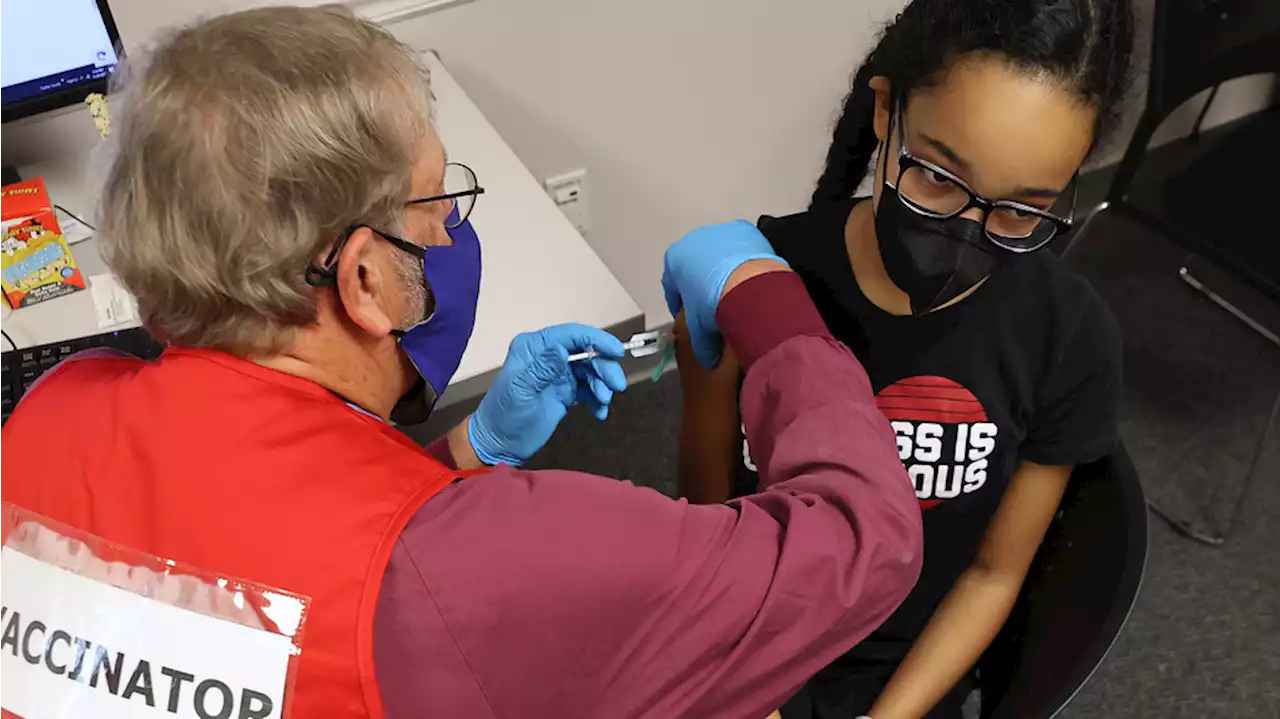
Pfizer will submit new data to the FDA this week about trials of its vaccine for kids younger than 5 years old. Here, an older child receives the Pfizer BioNTech COVID-19 vaccine in Virginia last November.
Pfizer's pediatric COVID-19 vaccine has an efficacy of 80.3%, according to a preliminary analysis, and meets"all immunobridging criteria required for Emergency Use Authorization," the company said Monday. The results are based on clinical trials in which kids from six months to age 5 got three doses of the company's vaccine.
We have summarized this news so that you can read it quickly. If you are interested in the news, you can read the full text here. Read more: