Pfizer asks the FDA to authorize an updated booster shot.
on a different version of a bivalent shot, one that targeted the original omicron variant. That booster was shown to be safe and produced an immune response.
Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief, said it “makes sense” to update the vaccines to target new variants circulating. It is “likely” the immune responses in people will be similar to those seen in the animal studies, he said, but “it is not known until human data are available.”
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Variant-Targeted Covid-19 Boosters Test the Promise of mRNA TechnologyThe race to produce variant-targeted Covid-19 boosters for the U.S. is a test of the plug-and-play potential of Moderna’s and Pfizer’s MRNA technology
Variant-Targeted Covid-19 Boosters Test the Promise of mRNA TechnologyThe race to produce variant-targeted Covid-19 boosters for the U.S. is a test of the plug-and-play potential of Moderna’s and Pfizer’s MRNA technology
Read more »
 Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives
Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives
Read more »
 Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Pfizer seeks OK of updated COVID vaccine booster for fallPfizer has asked U.S. regulators to authorize its combination COVID-19 vaccine that adds protection against the newest omicron relatives.
Read more »
 FDA Authorizes Emergency Use for Novavax Covid-19 Vaccine for Ages 12 to 17Novavax announced on Saturday that its COVID-19 vaccine has been authorized for emergency use by the FDA for adolescents between the ages of 12 and 17.
FDA Authorizes Emergency Use for Novavax Covid-19 Vaccine for Ages 12 to 17Novavax announced on Saturday that its COVID-19 vaccine has been authorized for emergency use by the FDA for adolescents between the ages of 12 and 17.
Read more »
 What to know about polio boosters, oral vaccines, and your medical history recordsWith new polio cases surfacing in New York, health officials are sharing guidance on who should and shouldn't get a polio vaccine booster shot.
What to know about polio boosters, oral vaccines, and your medical history recordsWith new polio cases surfacing in New York, health officials are sharing guidance on who should and shouldn't get a polio vaccine booster shot.
Read more »
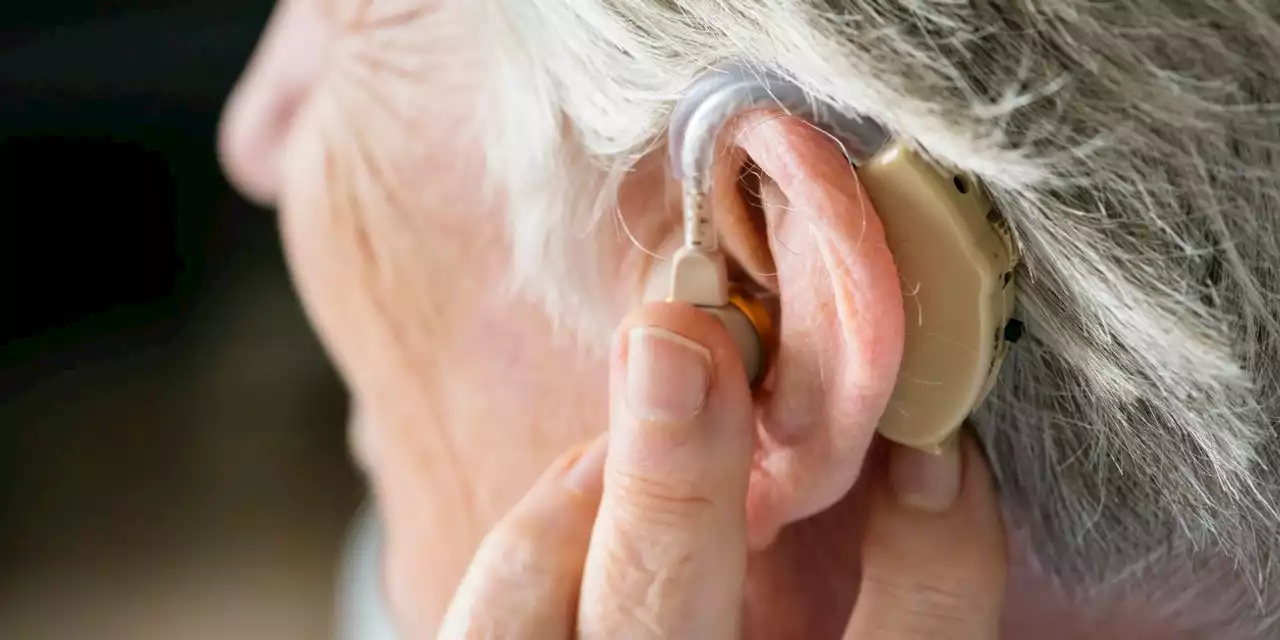 Over-the-counter hearing aids may be available in the U.S. as soon as OctoberOver-the-counter hearing aids got FDA approval. Millions of Americans will have access to more affordable hearing aids by mid-October when the rule takes effect.
Over-the-counter hearing aids may be available in the U.S. as soon as OctoberOver-the-counter hearing aids got FDA approval. Millions of Americans will have access to more affordable hearing aids by mid-October when the rule takes effect.
Read more »