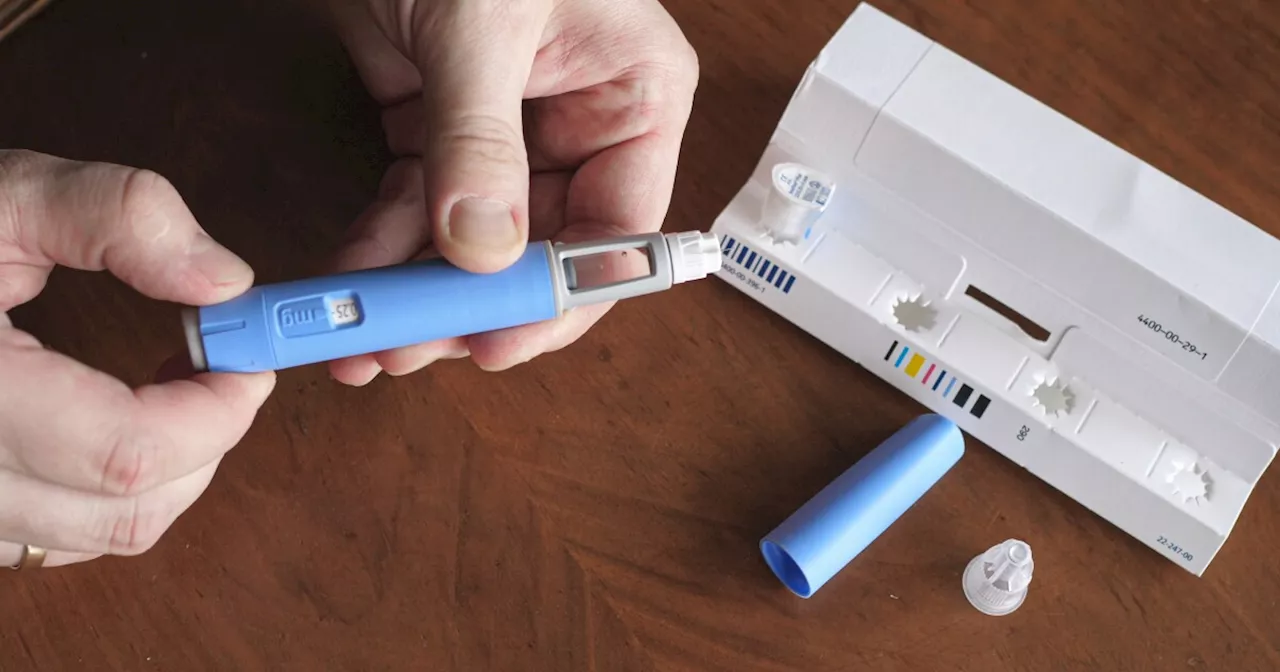
Those taking compounded versions are self-administering doses, and some reported administering 5 to 20 times more than the intended amount, the FDA said.
People are overdosing on the compounded versions of semaglutide drugs like Ozempic and Wegovy, the U.S. Food and Drug Administration said Friday.
In an alert on Friday, the FDA said it had received adverse reports, including some requiring hospitalization, in which patients mistakenly injected themselves with 5 to 20 times more than the intended dose of semaglutide. The overdose reports described severe symptoms like nausea, vomiting, fainting, migraine, dehydration, low blood sugar, acute pancreatitis and gallstones. And because of the one-week half-life of semaglutide products, the symptoms could last a while, requiring a prolonged observation period, the FDA said.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Overdoses on Ozempic Copies So Common, The FDA Had to Issue a WarningThe Best in Science News and Amazing Breakthroughs
Overdoses on Ozempic Copies So Common, The FDA Had to Issue a WarningThe Best in Science News and Amazing Breakthroughs
Read more »
 Ozempic May Lower Dementia Risk in People With Type 2 Diabetes, Study FindsLooking for ways to improve your memory and focus? It could be as simple as chatting with a friend or picking up sudoku.
Ozempic May Lower Dementia Risk in People With Type 2 Diabetes, Study FindsLooking for ways to improve your memory and focus? It could be as simple as chatting with a friend or picking up sudoku.
Read more »
 Ozempic's popularity leads to shortages for people with Type 2 diabetesDrugs like Ozempic, Wegovy and Mounjaro are in such high demand that many patients with Type 2 diabetes can't get them when they need them.
Ozempic's popularity leads to shortages for people with Type 2 diabetesDrugs like Ozempic, Wegovy and Mounjaro are in such high demand that many patients with Type 2 diabetes can't get them when they need them.
Read more »
 Ozempic's popularity leads to shortages for people with Type 2 diabetesDrugs like Ozempic, Wegovy and Mounjaro are in such high demand that many patients with Type 2 diabetes can't get them when they need them.
Ozempic's popularity leads to shortages for people with Type 2 diabetesDrugs like Ozempic, Wegovy and Mounjaro are in such high demand that many patients with Type 2 diabetes can't get them when they need them.
Read more »
 Some people are overdosing on semaglutide, FDA warns | What you need to knowThe FDA says people may be subject to dosing errors.
Some people are overdosing on semaglutide, FDA warns | What you need to knowThe FDA says people may be subject to dosing errors.
Read more »
 Some people are overdosing on semaglutide, FDA warns | What you need to knowThe FDA says people may be subject to dosing errors.
Some people are overdosing on semaglutide, FDA warns | What you need to knowThe FDA says people may be subject to dosing errors.
Read more »