Emergent BioSolutions stock gains after FDA approves the company's opioid overdose-reversing nasal spray, Narcan, for over-the-counter sales.
Emergent BioSolutions Inc. announced Wednesday that the U.S. Food and Drug Administration approved its opioid overdose-reversing nasal spray, Narcan, for over-the-counter sales.
The news initially sent the drugmaker’s stock EBS soaring as much as 20%, but it has given up most of its gains to be up 6% in midday trading. The FDA’s approval was expected, as an FDA advisory committee had voted unanimously last month in favor of allowing over-the-counter sales of Narcan. “Today’s landmark FDA OTC approval for Narcan Nasal Spray marks a historic milestone as we have delivered on our commitment to make this important emergency treatment widely accessible, given the alarming rates of opioid overdoses occurring across the country,” said Chief Executive Officer Robert Kramer.
Emergent has said since its quarterly filing in November 2022 that “there is substantial doubt about the company’s ability to continue as a going concern within one year after the date that the financial statements are issued.”
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves over-the-counter sales of lifesaving opioid overdose treatment Narcan nasal sprayThe FDA's decision means people will be able to buy the 4mg nasal spray without a prescription in supermarkets, convenience stores and vending machines.
FDA approves over-the-counter sales of lifesaving opioid overdose treatment Narcan nasal sprayThe FDA's decision means people will be able to buy the 4mg nasal spray without a prescription in supermarkets, convenience stores and vending machines.
Read more »
 FDA approves first over-the-counter opioid overdose antidote Narcan | CNNWith drug overdose deaths continuing to hover near record levels, the US Food and Drug Administration on Wednesday approved for the first time an over-the-counter version of the opioid antidote Narcan.
FDA approves first over-the-counter opioid overdose antidote Narcan | CNNWith drug overdose deaths continuing to hover near record levels, the US Food and Drug Administration on Wednesday approved for the first time an over-the-counter version of the opioid antidote Narcan.
Read more »
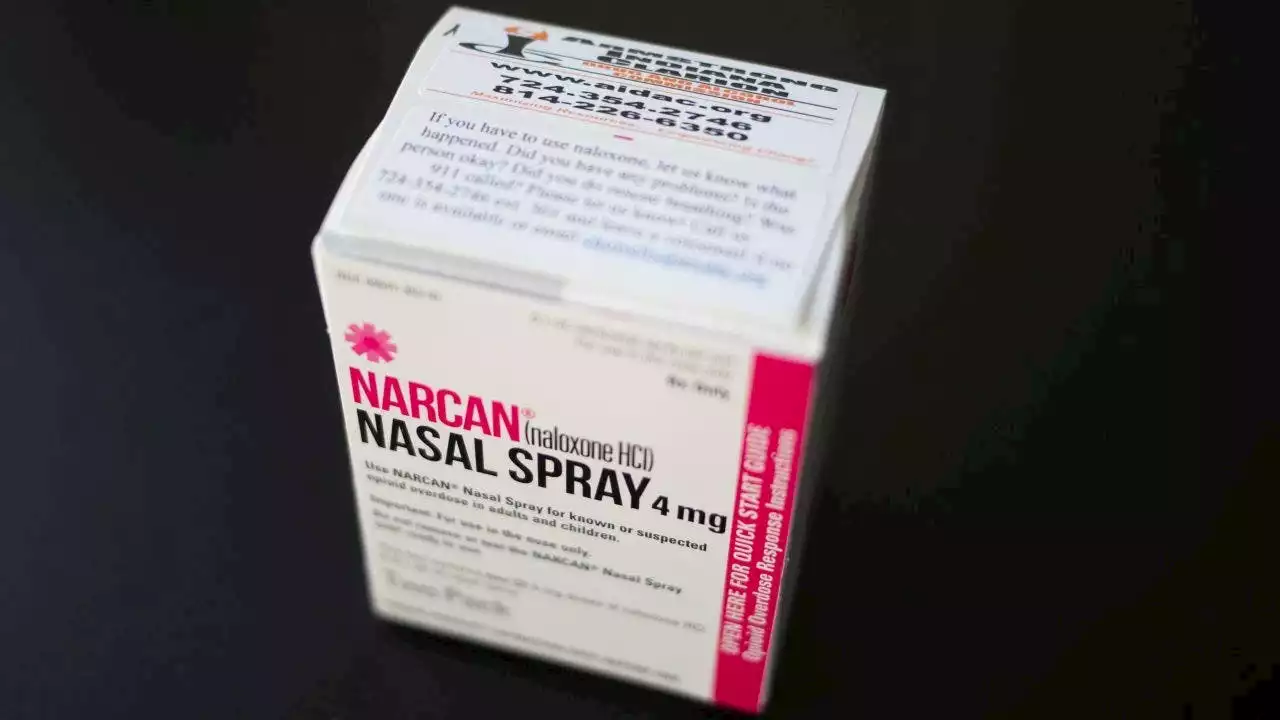 Opioid overdose drug Narcan approved for over-the-counter use by FDAThe U.S. Food and Drug Administration announced Wednesday it approved selling the life-saving naloxone-based nasal spray Narcan without a prescription.
Opioid overdose drug Narcan approved for over-the-counter use by FDAThe U.S. Food and Drug Administration announced Wednesday it approved selling the life-saving naloxone-based nasal spray Narcan without a prescription.
Read more »
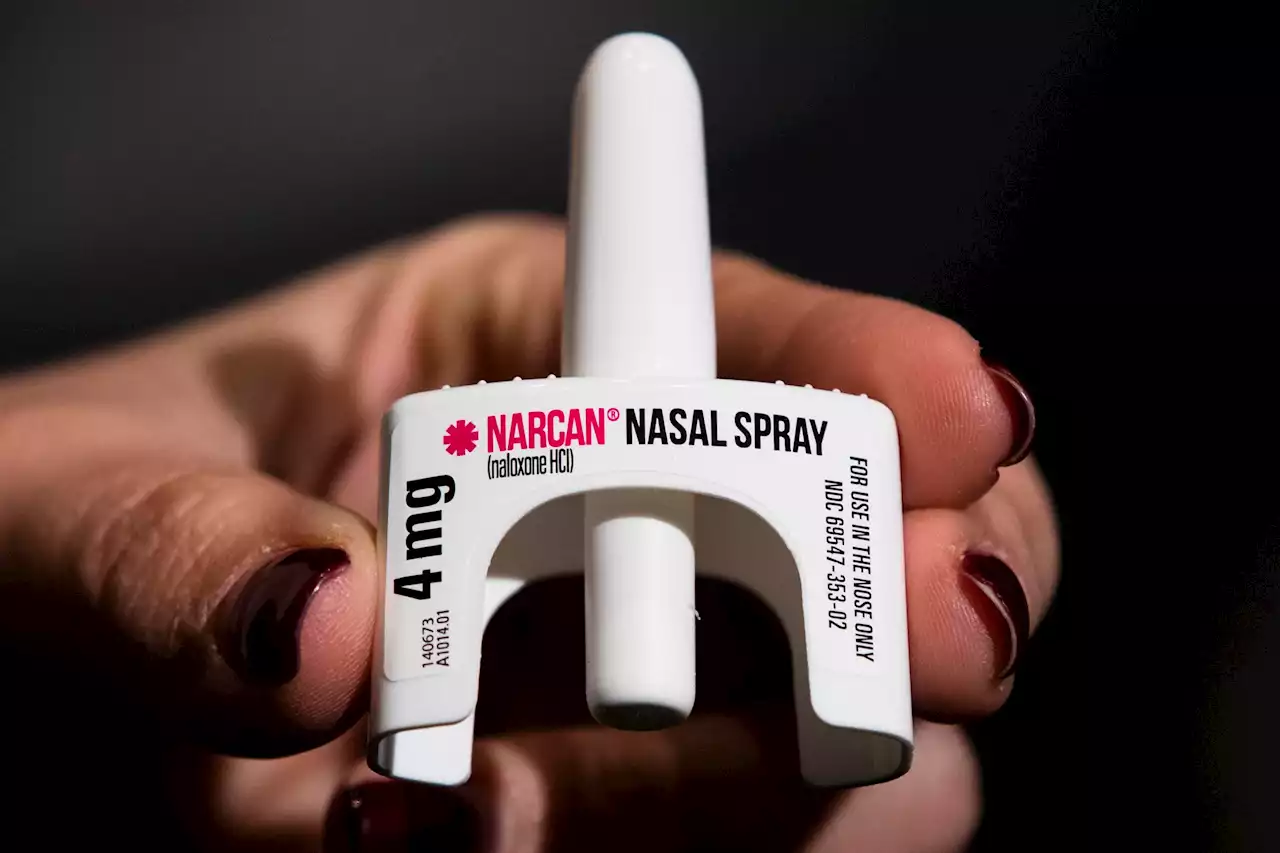 FDA Approves Narcan—An Opioid Antidote—For Over-The-Counter Use Amid Overdose EpidemicManufacturers will determine the timeline and price of the drug, the FDA said.
FDA Approves Narcan—An Opioid Antidote—For Over-The-Counter Use Amid Overdose EpidemicManufacturers will determine the timeline and price of the drug, the FDA said.
Read more »
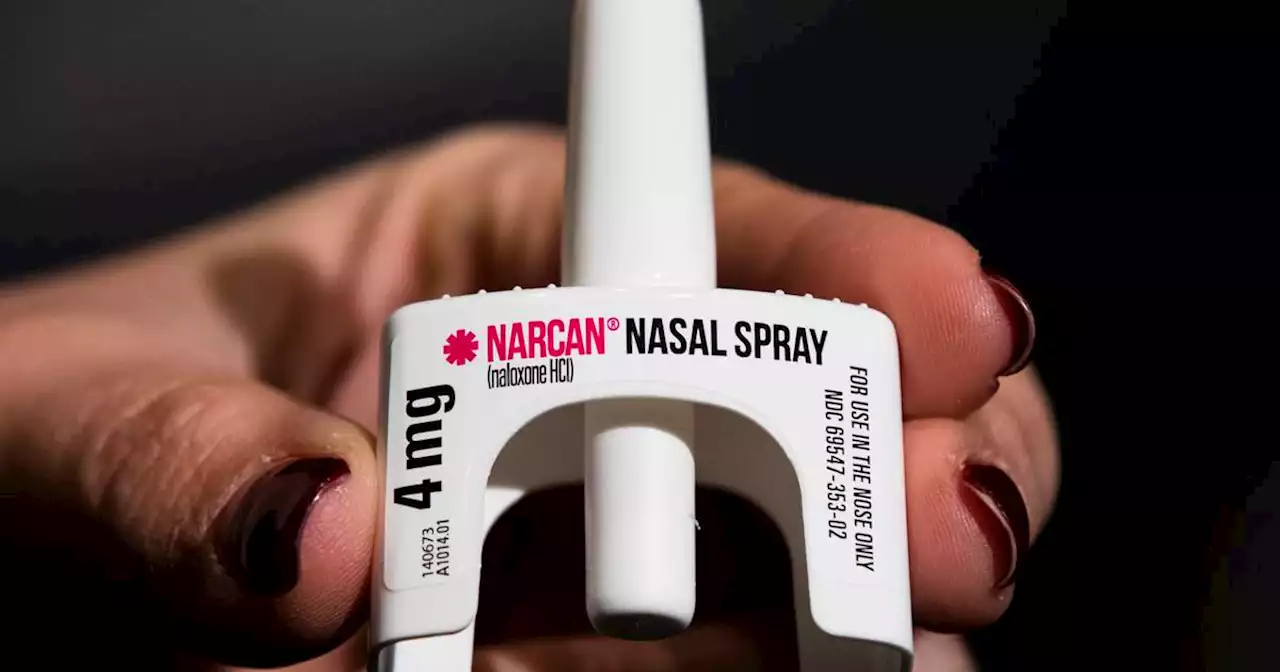 FDA approves over-the-counter sales of lifesaving Narcan for opioid overdosesThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
FDA approves over-the-counter sales of lifesaving Narcan for opioid overdosesThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
Read more »
