Vertex Pharmaceuticals' Journavx, a nonaddictive drug for moderate to severe acute pain, has been approved by the FDA. Experts believe it could reduce opioid prescriptions after surgery or for patients who can't tolerate other pain medications. While initial trials show promising results, some experts call for further research.
The drug, Journavx, from Vertex Pharmaceuticals, reduced pain after surgery in clinical trials. Experts hope it can lead to fewer opioid prescriptions.
The body registers pain through nerve endings throughout the body. Touch a hot stove, for example, and the nerve will send signals to the spinal cord and up to the brain that you’re experiencing pain in your hand. Opioids work by stimulating opioid receptors in the brain, blocking those pain signals. During the process, the brain also floods with the neurotransmitter dopamine, creating feelings of euphoria and activating the brain’s reward system.
Some of the patients who got suzetrigine also took ibuprofen as a so-called rescue medication — that is, if they were still experiencing pain after their suzetrigine doses. About 50% of people in the tummy tuck group and about 30% in the bunion surgery group reported some kind of side effect, most commonly headache, constipation or nausea, but, except for constipation, the side effects were less common in patients who got suzetrigine compared with an opioid.
While the clinical trial showed the drug could be effective as a solo treatment for pain — though it was more effective when patients combined it with ibuprofen — suzetrigine is meant to be used as part of a step-up approach, after Tylenol and NSAIDs, Bertoch said. In those situations, “you are willing to accept a higher risk,” Rind said. “With a new pain reliever, it really has to be incredibly safe to be OK, and we really will not know that until it’s on the market and used by lots of people.”
Schatman, of NYU Grossman School of Medicine, said suzetrigine does seem safe and effective for short-term use; however, he voiced concerns that Vertex doesn’t have longer-term safety data.
PAIN MANAGEMENT OPIOIDS NEW DRUG SURGERY FDA APPROVAL
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Journavx: A Promising New Drug for Acute Pain ReliefVertex Pharmaceuticals' Journavx, a non-addictive pain reliever, has shown significant promise in clinical trials, potentially reducing reliance on opioids for acute pain management. The FDA approved drug targets a specific sodium channel, blocking pain signals from reaching the brain, offering a safer alternative for patients.
Journavx: A Promising New Drug for Acute Pain ReliefVertex Pharmaceuticals' Journavx, a non-addictive pain reliever, has shown significant promise in clinical trials, potentially reducing reliance on opioids for acute pain management. The FDA approved drug targets a specific sodium channel, blocking pain signals from reaching the brain, offering a safer alternative for patients.
Read more »
 New Drug Journavx Offers Hope for Reduced Opioid Use After SurgeryJournavx, a nonaddictive drug developed by Vertex Pharmaceuticals, has shown promising results in clinical trials for reducing post-surgical pain. Experts believe it could significantly reduce the reliance on opioid painkillers.
New Drug Journavx Offers Hope for Reduced Opioid Use After SurgeryJournavx, a nonaddictive drug developed by Vertex Pharmaceuticals, has shown promising results in clinical trials for reducing post-surgical pain. Experts believe it could significantly reduce the reliance on opioid painkillers.
Read more »
 FDA Approves New Nonopioid Pain Killer JournavxThe FDA has approved a new nonopioid painkiller, Journavx, for moderate to severe acute pain in adults. This could potentially reduce opioid prescriptions after surgery or provide an alternative for those who can't tolerate other pain medications.
FDA Approves New Nonopioid Pain Killer JournavxThe FDA has approved a new nonopioid painkiller, Journavx, for moderate to severe acute pain in adults. This could potentially reduce opioid prescriptions after surgery or provide an alternative for those who can't tolerate other pain medications.
Read more »
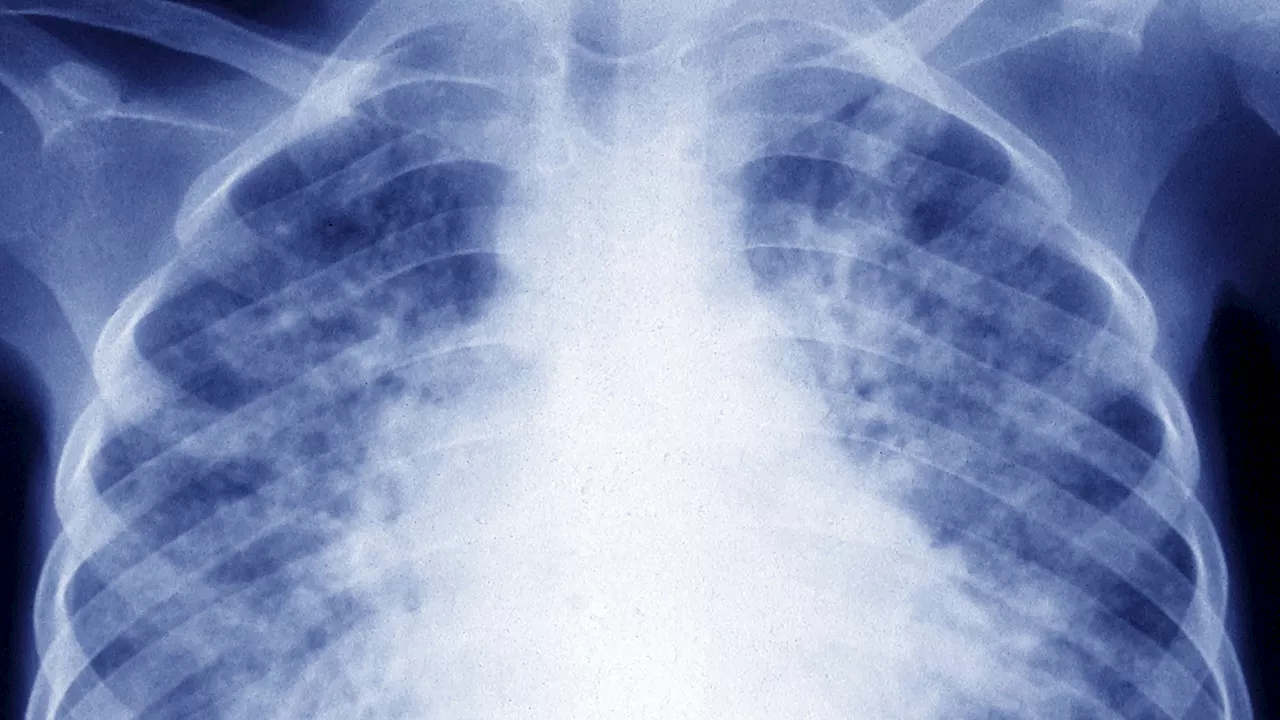 New Drug Regimens Offer Hope in Fight Against Drug-Resistant TuberculosisA groundbreaking international clinical trial has revealed three new, safe, and effective drug regimens for treating drug-resistant tuberculosis (TB). These advancements hold the potential to significantly improve access to life-saving care for individuals battling this deadly infectious disease.
New Drug Regimens Offer Hope in Fight Against Drug-Resistant TuberculosisA groundbreaking international clinical trial has revealed three new, safe, and effective drug regimens for treating drug-resistant tuberculosis (TB). These advancements hold the potential to significantly improve access to life-saving care for individuals battling this deadly infectious disease.
Read more »
 New Drug Regimens Offer Hope in Fight Against Drug-Resistant TBInternational clinical trial identifies three new regimens for rifampin-resistant TB, using recently discovered drugs to provide shorter, personalized treatment with fewer side effects. WHO recommends these regimens, boosting access to life-saving care for vulnerable populations.
New Drug Regimens Offer Hope in Fight Against Drug-Resistant TBInternational clinical trial identifies three new regimens for rifampin-resistant TB, using recently discovered drugs to provide shorter, personalized treatment with fewer side effects. WHO recommends these regimens, boosting access to life-saving care for vulnerable populations.
Read more »
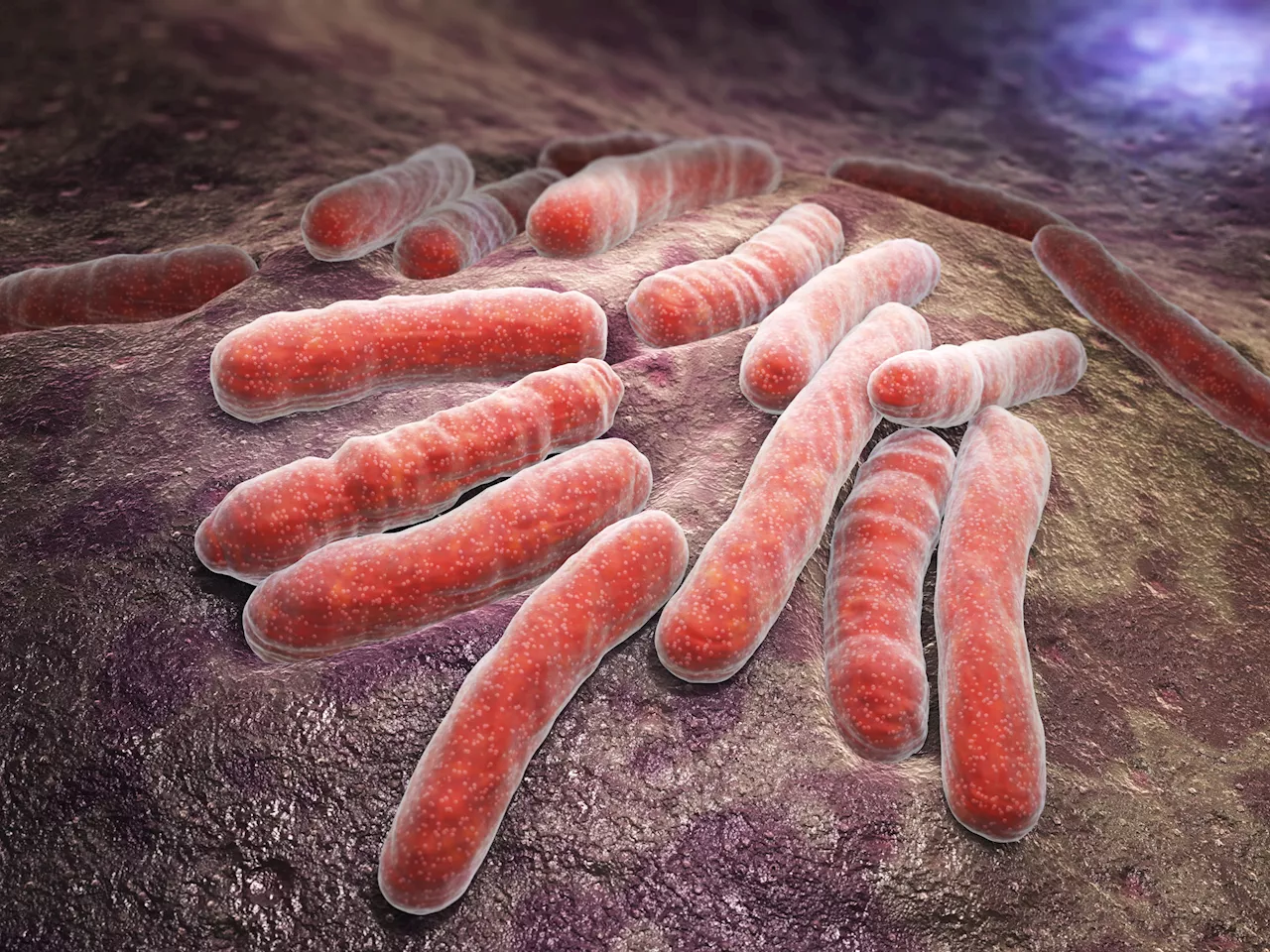 New Drug Regimens Offer Hope in Fight Against Drug-Resistant TuberculosisAn international clinical trial has discovered three highly effective drug regimens for treating tuberculosis resistant to rifampin, a key antibiotic used in TB treatment. These findings, published in the New England Journal of Medicine, could significantly improve patient outcomes and transform the fight against this global health crisis.
New Drug Regimens Offer Hope in Fight Against Drug-Resistant TuberculosisAn international clinical trial has discovered three highly effective drug regimens for treating tuberculosis resistant to rifampin, a key antibiotic used in TB treatment. These findings, published in the New England Journal of Medicine, could significantly improve patient outcomes and transform the fight against this global health crisis.
Read more »
