The U.S. Food and Drug Administration on Tuesday approved another drug that slows cognitive and functional decline in people with early symptomatic Alzheimer's disease, including mild cognitive impairment or mild dementia.
<p>SALT LAKE CITY — The U.S.
 In the phase 3 clinical trial, those receiving the drug declined cognitively about 35% more slowly than those receiving a placebo infusion, Eli Lilly reported in a <a href="https:/&#
Traffic Weather Sports Classifieds Cars Jobs Homes Television Radio Salt Lake Utah Local
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA advisors strongly back new Alzheimer’s drug, despite risks and limitationsAdvisors to the Food and Drug Administration have recommended unanimously that the agency approve the Alzheimer’s drug donanemab.
FDA advisors strongly back new Alzheimer’s drug, despite risks and limitationsAdvisors to the Food and Drug Administration have recommended unanimously that the agency approve the Alzheimer’s drug donanemab.
Read more »
 FDA advisors strongly back new Alzheimer’s drug, despite risks and limitationsAdvisors to the Food and Drug Administration have recommended unanimously that the agency approve the Alzheimer’s drug donanemab.
FDA advisors strongly back new Alzheimer’s drug, despite risks and limitationsAdvisors to the Food and Drug Administration have recommended unanimously that the agency approve the Alzheimer’s drug donanemab.
Read more »
 Independent FDA Panel Endorses New Alzheimer’s Disease TreatmentAn FDA advisory panel unanimously gave a green light on Monday to the Alzheimer’s disease treatment donanemab for use during early stages of the disease.
Independent FDA Panel Endorses New Alzheimer’s Disease TreatmentAn FDA advisory panel unanimously gave a green light on Monday to the Alzheimer’s disease treatment donanemab for use during early stages of the disease.
Read more »
 FDA approves new Alzheimer's treatment, donanemab from Eli LillyFDA approval of the new Alzheimer's treatment, which will be branded as Kisunla, follows years of setbacks.
FDA approves new Alzheimer's treatment, donanemab from Eli LillyFDA approval of the new Alzheimer's treatment, which will be branded as Kisunla, follows years of setbacks.
Read more »
 FDA approves new drug for Alzheimer’s disease: ‘Meaningful results’The FDA has approved a new medication for people with Alzheimer’s disease. Eli Lilly’s Kisunla (donanemab) is a once-monthly injection intended for adults with early symptomatic Alzheimer's disease.
FDA approves new drug for Alzheimer’s disease: ‘Meaningful results’The FDA has approved a new medication for people with Alzheimer’s disease. Eli Lilly’s Kisunla (donanemab) is a once-monthly injection intended for adults with early symptomatic Alzheimer's disease.
Read more »
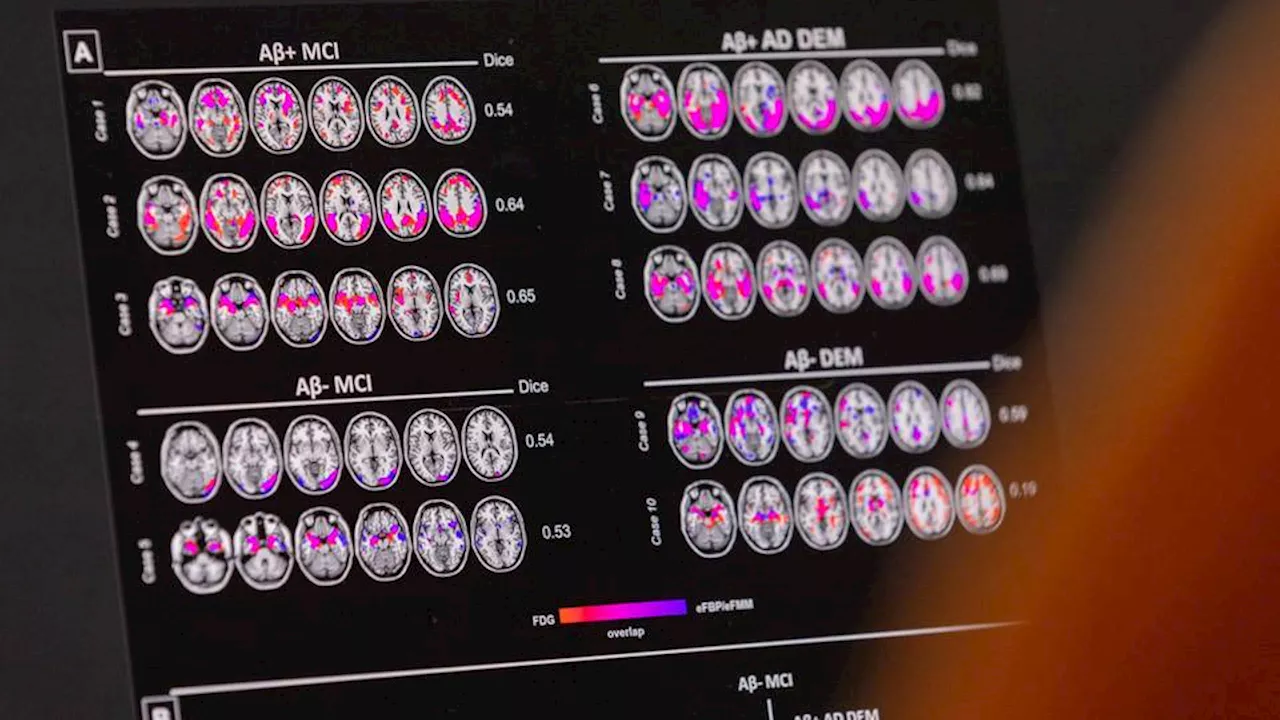 FDA approves new Alzheimer’s drug, expanding treatment options for patientsEli Lilly's donanemab has received FDA approval as only the second therapy of its kind, backed unanimously by top experts, endorsing its use in early-stage Alzheimer's patients and highlighting the drug's favourable risk-benefit profile.
FDA approves new Alzheimer’s drug, expanding treatment options for patientsEli Lilly's donanemab has received FDA approval as only the second therapy of its kind, backed unanimously by top experts, endorsing its use in early-stage Alzheimer's patients and highlighting the drug's favourable risk-benefit profile.
Read more »
