$158,000 a year. That's how much a new ALS treatment costs — and it just won FDA approval without the clinical trial evidence the agency usually requires.
But Aduhelm has serious side effects, raising the question of whether it was ethical to put the drug in patients' hands before it was even clear it could help them.
Such safety questions aren't part of the debate over Relyvrio, which the FDA says has "no significant safety signals of concern."Federal law now has the power to limit the prices of old drugs or the price increases of drugs already on the market, but can do nothing to constrain the launch prices of new drugs.But it's against this backdrop that the drug pricing debates of the future will play out.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves treatment that ALS Ice Bucket Challenge helped fundIn 2014, people all over the world joined in on the ALS ice bucket challenge. It raised $115 million in donations, according to the ALS Association.
FDA approves treatment that ALS Ice Bucket Challenge helped fundIn 2014, people all over the world joined in on the ALS ice bucket challenge. It raised $115 million in donations, according to the ALS Association.
Read more »
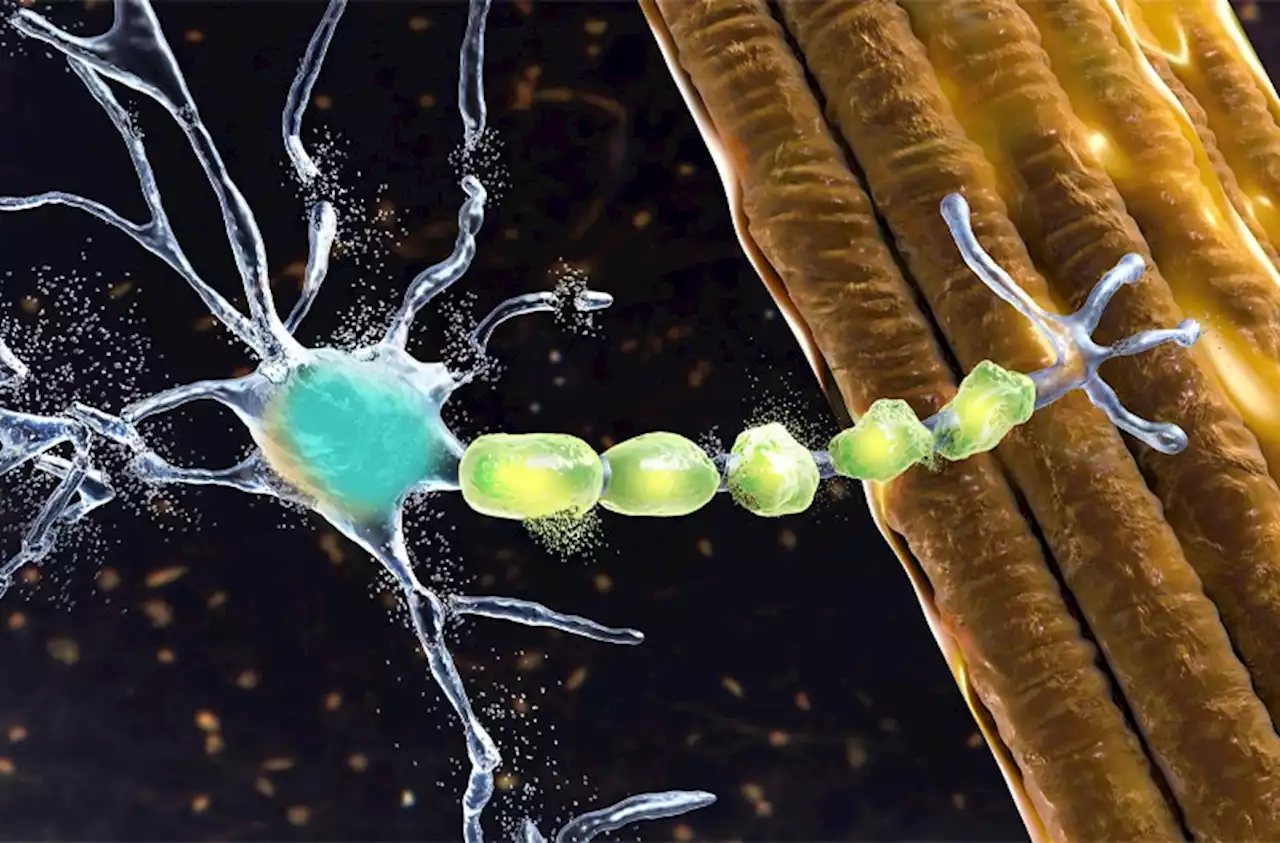 ALS therapy hits molecular mark but misses clinical one⭐ Research Highlight: In a phase 3 clinical trial, the antisense oligonucleotide therapy tofersen did not lead to clinical improvements in patients with ALS, despite reducing the amount of toxic SOD1 protein. NEJM
ALS therapy hits molecular mark but misses clinical one⭐ Research Highlight: In a phase 3 clinical trial, the antisense oligonucleotide therapy tofersen did not lead to clinical improvements in patients with ALS, despite reducing the amount of toxic SOD1 protein. NEJM
Read more »
 ALS drug wins FDA approval despite questionable dataWhen it comes to tradition it's not easy to create change, especially when that tradition comes from hundreds of years of history.
ALS drug wins FDA approval despite questionable dataWhen it comes to tradition it's not easy to create change, especially when that tradition comes from hundreds of years of history.
Read more »
 ‘There's no way that patients are going to be able to afford that.’ Why aren’t new drugs that can help you lose weight more widely used?‘There’s no way that patients are going to be able to afford that.’ Why aren’t new drugs that can help you lose weight more widely used?
‘There's no way that patients are going to be able to afford that.’ Why aren’t new drugs that can help you lose weight more widely used?‘There’s no way that patients are going to be able to afford that.’ Why aren’t new drugs that can help you lose weight more widely used?
Read more »
