The product was never submitted for approval by the FDA and is an unapproved drug for which safety and efficacy have not been established. n
has been issued for nasal solution products used in hospitals because of the potential for errors in administering the drug.
Both products are distributed to hospitals for use by healthcare professionals. The two labels are very similar, which makes it hard to distinguish between the non-sterile topical and sterile injectable product.
Adrenalin® Chloride Solution 30 mL vial is distributed in individually packed cartons under NDC #42023-103-01 with the words “Nasal Solution USP” and “For Topical Application” on the package.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Nasal Spray May Slow Alzheimer's Disease, Study in Mice SuggestsThe Best in Science News and Amazing Breakthroughs
Nasal Spray May Slow Alzheimer's Disease, Study in Mice SuggestsThe Best in Science News and Amazing Breakthroughs
Read more »
 FDA says decongestant in many cold medicines doesn't work. So what does?The most popular nasal decongestant on U.S. pharmacy shelves may not be there much longer.
FDA says decongestant in many cold medicines doesn't work. So what does?The most popular nasal decongestant on U.S. pharmacy shelves may not be there much longer.
Read more »
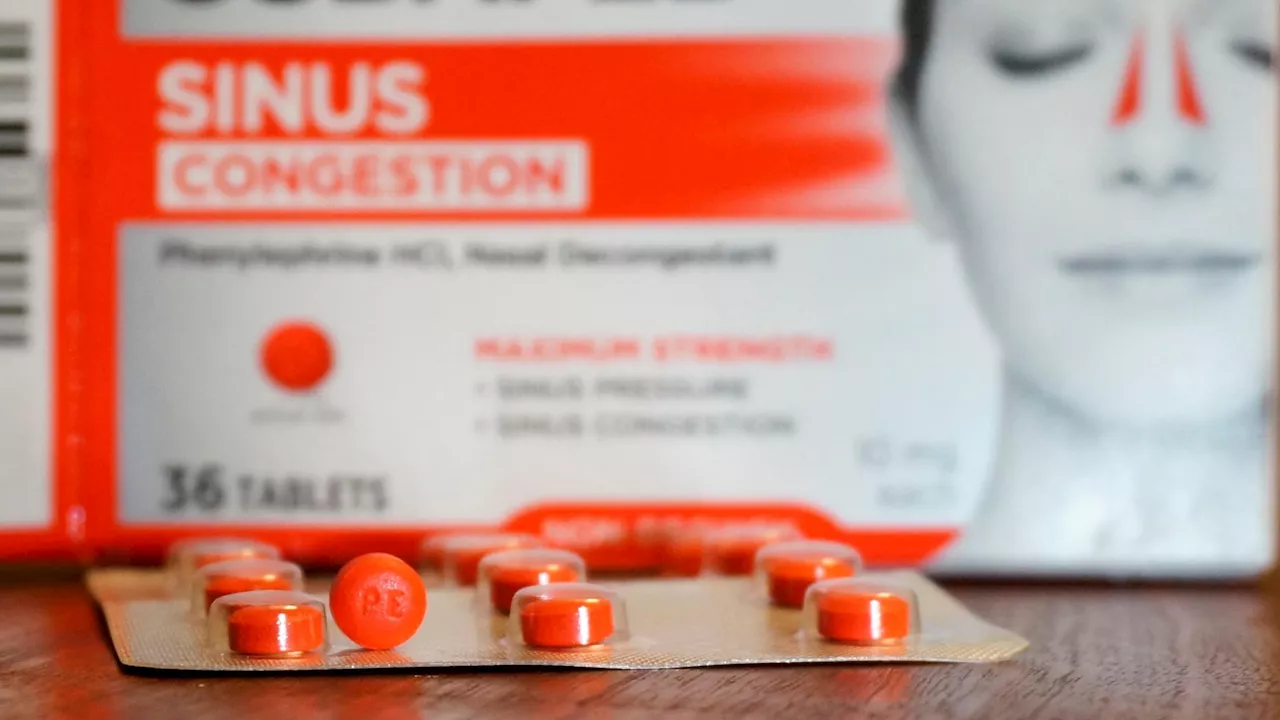 Popular Decongestant Phased Out for Lack of EffectivenessThe FDA is phasing out phenylephrine, the leading nasal decongestant found in hundreds of over-the-counter medications, due to its lack of proven effectiveness.
Popular Decongestant Phased Out for Lack of EffectivenessThe FDA is phasing out phenylephrine, the leading nasal decongestant found in hundreds of over-the-counter medications, due to its lack of proven effectiveness.
Read more »
 FDA to Phase Out Common DecongestantThe FDA is taking action to remove phenylephrine, a common decongestant found in many over-the-counter medications, due to its lack of effectiveness in relieving nasal congestion.
FDA to Phase Out Common DecongestantThe FDA is taking action to remove phenylephrine, a common decongestant found in many over-the-counter medications, due to its lack of effectiveness in relieving nasal congestion.
Read more »
 US to Ban Common Decongestant Found IneffectiveThe FDA is proposing to ban phenylephrine, a common decongestant found in many over-the-counter medications, after concluding it doesn't actually relieve nasal congestion.
US to Ban Common Decongestant Found IneffectiveThe FDA is proposing to ban phenylephrine, a common decongestant found in many over-the-counter medications, after concluding it doesn't actually relieve nasal congestion.
Read more »
 FDA Moves to Ban Common Decongestant, PhenylephrineThe U.S. Food and Drug Administration (FDA) is phasing out phenylephrine, the leading decongestant found in hundreds of over-the-counter medications, due to a lack of effectiveness in relieving nasal congestion. This decision, based on decades of research and unanimous votes from FDA advisors, will likely force drugmakers to reformulate or remove products containing phenylephrine. Consumers may need to switch to alternative decongestants, potentially including those requiring a pharmacy prescription.
FDA Moves to Ban Common Decongestant, PhenylephrineThe U.S. Food and Drug Administration (FDA) is phasing out phenylephrine, the leading decongestant found in hundreds of over-the-counter medications, due to a lack of effectiveness in relieving nasal congestion. This decision, based on decades of research and unanimous votes from FDA advisors, will likely force drugmakers to reformulate or remove products containing phenylephrine. Consumers may need to switch to alternative decongestants, potentially including those requiring a pharmacy prescription.
Read more »
