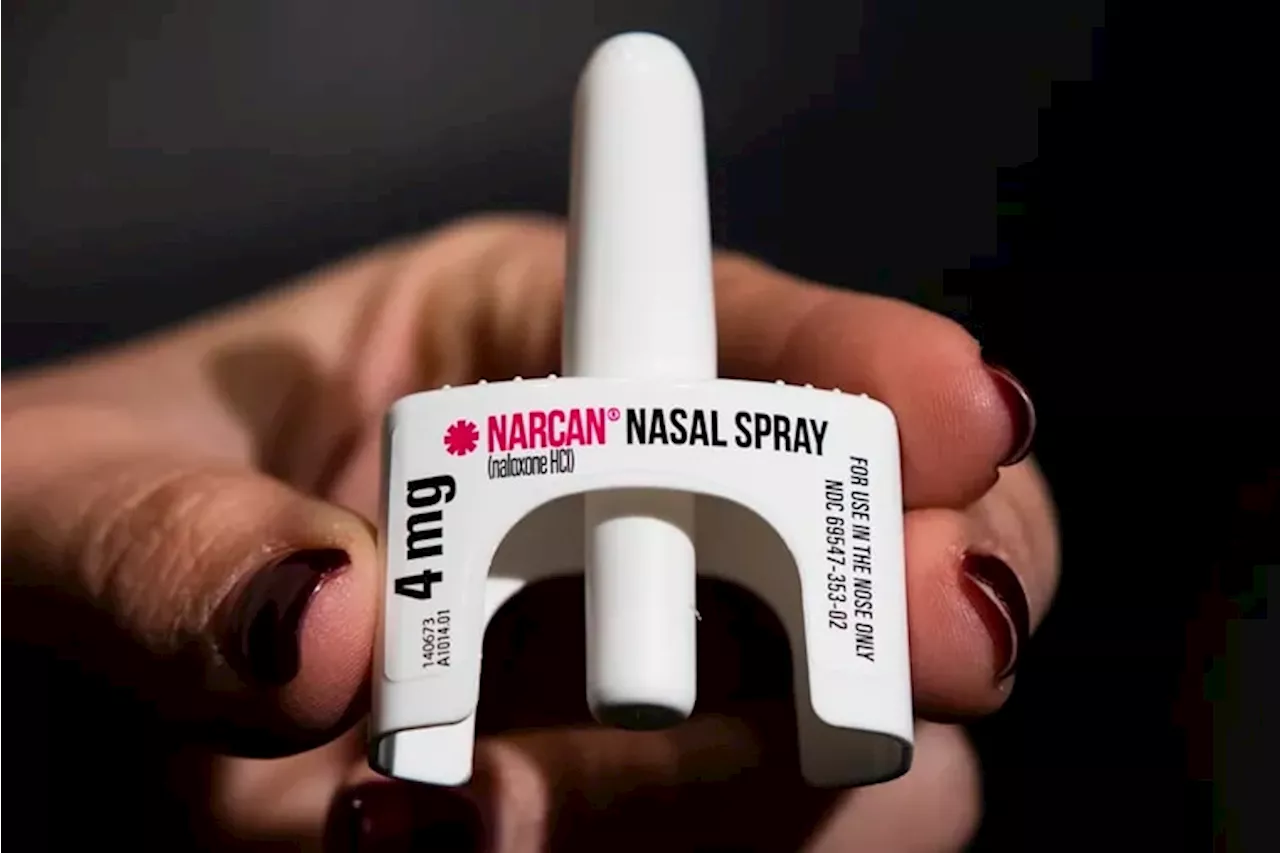
The FDA approved over-the-counter marketing and sales of Narcan in March.
Last month, drugstores and pharmacies nationwide began stocking and selling the country’s first over-the-counter version of naloxone, a medication that can stop a potentially fatal overdose from opioids. It’s sold as a nasal spray under the brand name Narcan.
“For those who don’t have substance use concerns, they might go in and just ask for the product and not be concerned about what the other person’s thinking,” he said. “But that’s a mental state that’s very hard for most of us to put ourselves into if we don’t live the life of somebody with the stigma and the marginalization that is so associated with substance use.”
“That’s a lot of money to be spending on something if you need food today, if you have a headache and need ibuprofen today,” she said. “You think you’ll probably need naloxone, but it’s not a guarantee that you’ll need today, so why spend the money?”For some consumers, purchasing naloxone via prescription could remain cheaper than buying it over the counter. Many private health insurers — and public programs like Medicaid and Medicare — cover the cost of these prescription sales.
One common misconception Hernandez runs into surrounds Narcan’s packaging, which says “nasal spray” in large letters on the box. It’s not always clear if someone is experiencing an overdose, but Hernandez told the group that they should still call 911 and administer Narcan.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA gathering information about woman who died after allegedly drinking Panera Bread beverageAccording to Katz’s autopsy report, her cause of death was listed as cardiac arrhythmia due to long QT syndrome.
FDA gathering information about woman who died after allegedly drinking Panera Bread beverageAccording to Katz’s autopsy report, her cause of death was listed as cardiac arrhythmia due to long QT syndrome.
Read more »
 FDA warns against giving probiotics to babies after infant deathBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA warns against giving probiotics to babies after infant deathBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Read more »
 FDA warns about giving probiotics to preterm babies after infant death, other injuriesFederal officials are warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products in the hospital. The FDA says that one death this year and more than two dozen reports of other injuries since 2018 may be tied to the supplements.
FDA warns about giving probiotics to preterm babies after infant death, other injuriesFederal officials are warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products in the hospital. The FDA says that one death this year and more than two dozen reports of other injuries since 2018 may be tied to the supplements.
Read more »
 FDA warns about giving probiotics to preterm babies after infant death, other injuriesFederal officials are warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products in the hospital.
FDA warns about giving probiotics to preterm babies after infant death, other injuriesFederal officials are warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products in the hospital.
Read more »
 FDA warns about giving probiotics to preterm babies after infant death, other injuriesFederal officials are warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products in the hospital.
FDA warns about giving probiotics to preterm babies after infant death, other injuriesFederal officials are warning health care providers and the public about injuries and at least one death in premature infants who were given probiotic products in the hospital.
Read more »