“This is reassuring data,” says Erin Longbrake, an assistant professor of neurology at the Yale School of Medicine. “We want to give these vulnerable patients every layer of protection that we can.”
of the mRNA vaccines for COVID-19 focused on healthy volunteers. People whose health was frail due to cancer or other serious illnesses face a heightened risk of complications from COVID-19 but weren’t included in these studies. This led some to hesitate about getting vaccinated once the shots were authorized due to concerns about side effects or fears that the immune response prompted by the vaccine would worsen their disease.
About 77 percent of the participants reported experiencing side effects after the first dose, including 11 percent who described their symptoms as severe. After the second dose about 66 percent of participants reported side effects, and about 15 percent said their symptoms were severe. The most frequent side effects were pain at the injection site, fatigue, bone pain, headaches, and fever. Other symptoms included nausea, diarrhea, insomnia, skin rashes, or enlarged lymph nodes.
“Whatever the underlying disease…the mRNA-COVID-19 vaccine should be considered a crucial step to allow a safe program of treatment more than a possible obstacle or danger to pursue the control of the disease,” Silvestris and his team concluded in the study.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Covid-19: Three Covid-related deaths and 2,054 casesThe total number of deaths linked to the virus reported by Stormont's Department of Health is 3,266.
Covid-19: Three Covid-related deaths and 2,054 casesThe total number of deaths linked to the virus reported by Stormont's Department of Health is 3,266.
Read more »
 Pfizer asks US to allow 4th COVID vaccine dose for seniorsPfizer and its partner BioNTech asked U.S. regulators to authorize an additional booster dose of their COVID-19 vaccine for seniors.
Pfizer asks US to allow 4th COVID vaccine dose for seniorsPfizer and its partner BioNTech asked U.S. regulators to authorize an additional booster dose of their COVID-19 vaccine for seniors.
Read more »
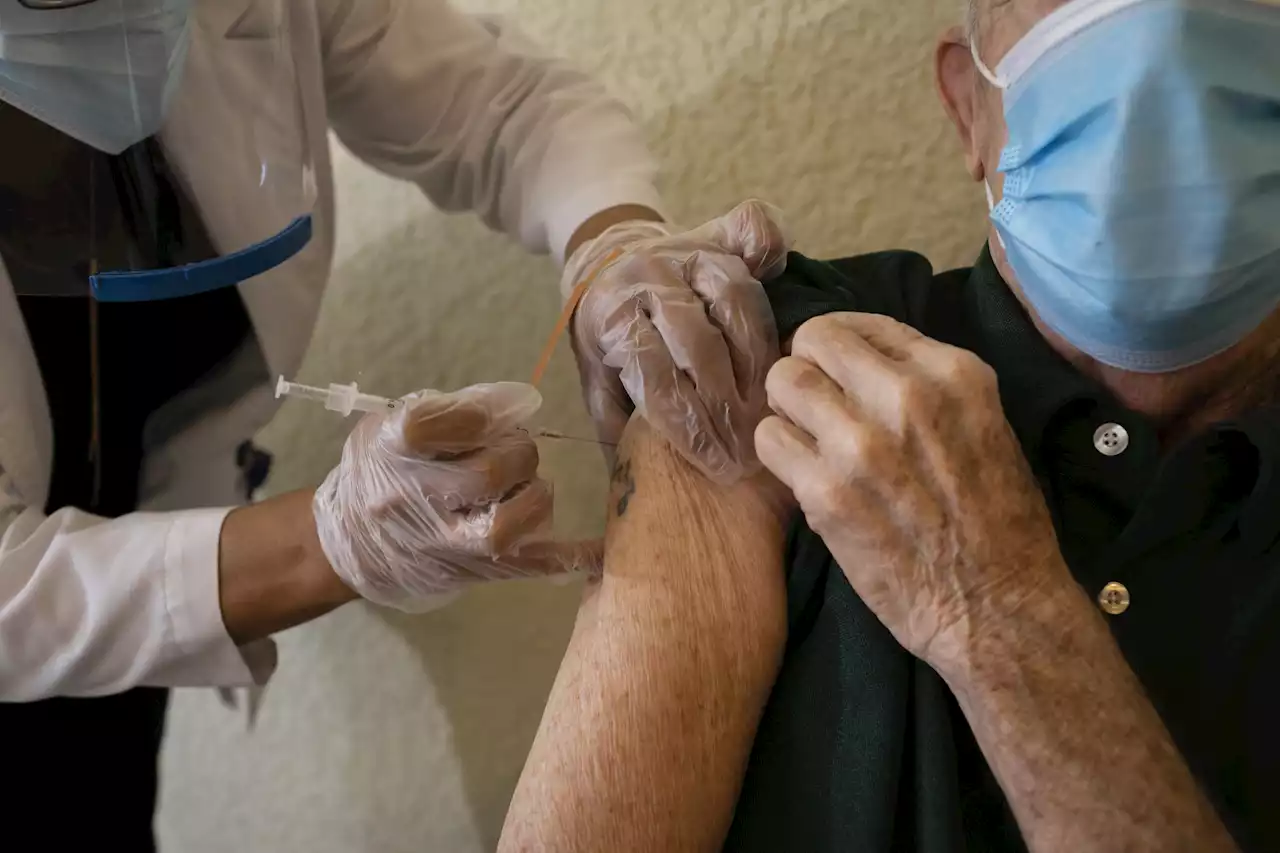 Pfizer Reportedly Set To Request Authorization For Second Covid Booster Shot For SeniorsPfizer-BioNTech will soon request FDA emergency authorization for a second Covid booster shot for people 65 and older, a group for which the agency has previously been quick to approve new shots, the Washington Post reported.
Pfizer Reportedly Set To Request Authorization For Second Covid Booster Shot For SeniorsPfizer-BioNTech will soon request FDA emergency authorization for a second Covid booster shot for people 65 and older, a group for which the agency has previously been quick to approve new shots, the Washington Post reported.
Read more »
 Pfizer asks US to authorize 4th COVID-19 vaccine dose for seniorsPfizer and its partner BioNTech asked U.S. regulators Tuesday to authorize an additional booster dose of their COVID-19 vaccine for seniors, saying data from Israel suggests older adults would benefit.
Pfizer asks US to authorize 4th COVID-19 vaccine dose for seniorsPfizer and its partner BioNTech asked U.S. regulators Tuesday to authorize an additional booster dose of their COVID-19 vaccine for seniors, saying data from Israel suggests older adults would benefit.
Read more »
 Pfizer-BioNTech will seek authorization for second COVID booster for older adultsPfizer is planning to ask the Food and Drug Administration to authorize a second COVID-19 booster shot for people 65 and older.
Pfizer-BioNTech will seek authorization for second COVID booster for older adultsPfizer is planning to ask the Food and Drug Administration to authorize a second COVID-19 booster shot for people 65 and older.
Read more »
 Pfizer COVID antiviral: Dozens of companies to start making pillNearly three dozen companies in Asia, the Caribbean, the Middle East and Eastern Europe will soon start making either generic versions of Pfizer's coronavirus pill or the raw ingredients. This deal will make the antiviral nirmatrelvir, or Paxlovoid, available to more than half of the world's population.
Pfizer COVID antiviral: Dozens of companies to start making pillNearly three dozen companies in Asia, the Caribbean, the Middle East and Eastern Europe will soon start making either generic versions of Pfizer's coronavirus pill or the raw ingredients. This deal will make the antiviral nirmatrelvir, or Paxlovoid, available to more than half of the world's population.
Read more »
