Moderna is the first company to release positive phase three data on a Covid and flu combination shot, giving it a lead over rivals Pfizer and Novavax.
Moderna said its combination vaccine targeting Covid and the flu was more effective than existing standalone shots for those viruses in a late-stage trial.
Moderna plans to file for regulatory approval for its combination jab this summer in the U.S. and hopes it can enter the market in 2025, said the company's CEO Stephane Bancel.that targets both Covid-19 and the flu was more effective than existing standalone shots for those viruses in a late-stage trial.Moderna plans to file for regulatory approval for its combination jab this summer in the U.S.
Bancel added that combination shots could reduce the burden of respiratory viruses on pharmacists and the broader U.S. health-care system, which has been grappling with a labor shortage that has many workers stretched thin.Moderna's messenger RNA combination shot, called mRNA-1083, is made up of both the company's vaccine candidate for seasonal influenza and a newer,"next-generation" version of its Covid shot.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Moderna and Pfizer competing for HHS bid on mRNA vaccines for bird fluModerna and Pfizer are both competing for federal contracts with HHS to contribute to an mRNA vaccine stockpile in case the H5N1 virus spreads to humans.
Moderna and Pfizer competing for HHS bid on mRNA vaccines for bird fluModerna and Pfizer are both competing for federal contracts with HHS to contribute to an mRNA vaccine stockpile in case the H5N1 virus spreads to humans.
Read more »
 Pfizer's Paxlovid fails as 15-day treatment for long COVID, study findsA study by Stanford University researchers found that a 15-day course of Pfizer's Paxlovid did not relieve symptoms of long COVID among 155 participants.
Pfizer's Paxlovid fails as 15-day treatment for long COVID, study findsA study by Stanford University researchers found that a 15-day course of Pfizer's Paxlovid did not relieve symptoms of long COVID among 155 participants.
Read more »
 Novavax stock jumps 50% as Sanofi deal kicks off turning point for struggling vaccine makerNovavax said the deal with Sanofi allows it to remove its 'going concern' warning, which it first issued last year due to doubts about staying afloat.
Novavax stock jumps 50% as Sanofi deal kicks off turning point for struggling vaccine makerNovavax said the deal with Sanofi allows it to remove its 'going concern' warning, which it first issued last year due to doubts about staying afloat.
Read more »
 Moderna and this solar name are among the most overbought stocks after a record-setting weekCertain stocks this week became overbought, based on their 14-day relative strength index, or RSI.
Moderna and this solar name are among the most overbought stocks after a record-setting weekCertain stocks this week became overbought, based on their 14-day relative strength index, or RSI.
Read more »
 Salesforce, Kohl's and Agilent fall premarket; Foot Locker, Moderna riseSalesforce, Kohl's and Agilent fall premarket; Foot Locker, Moderna rise
Salesforce, Kohl's and Agilent fall premarket; Foot Locker, Moderna riseSalesforce, Kohl's and Agilent fall premarket; Foot Locker, Moderna rise
Read more »
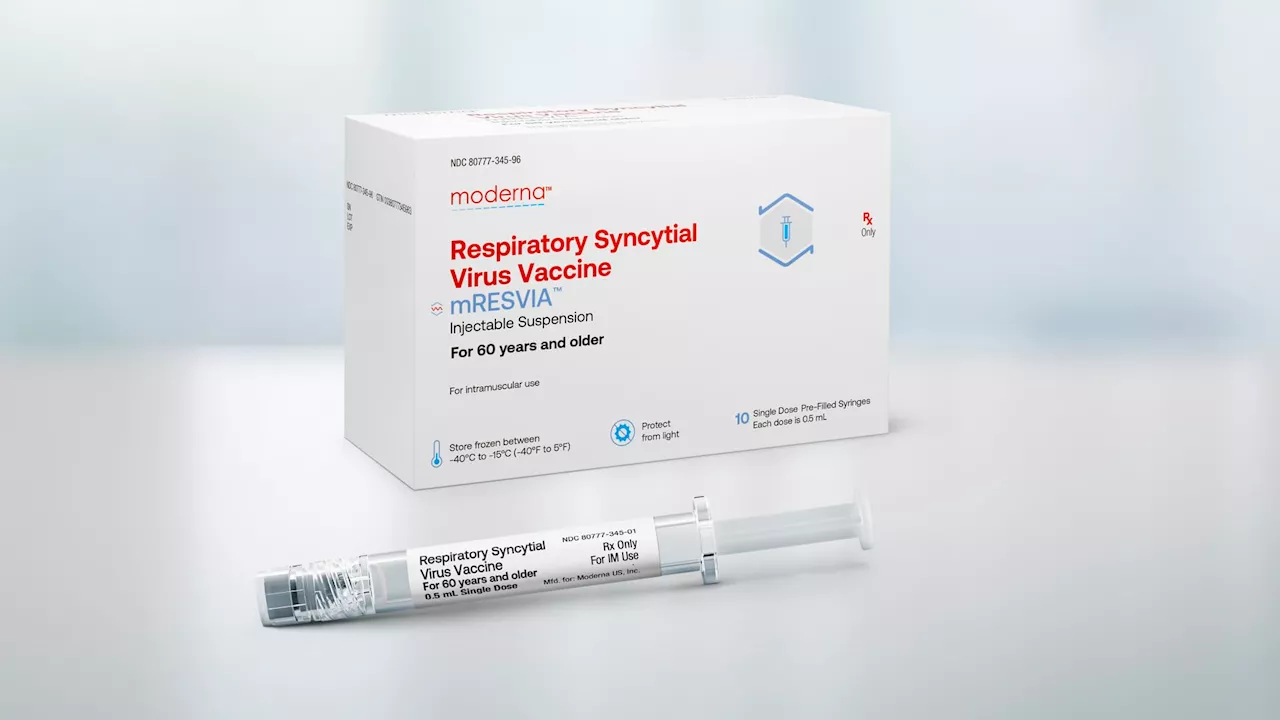 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »