But the timeline for FDA approval is still up in air.
A Moderna official said that it will take until about May 9 to get all of the paperwork to the government agency, and then it’s on the FDA to make a decision.: "We believe mRNA-1273 will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against COVID-19 and will be especially welcomed by parents and caregivers."
Moderna said that the lower effectiveness levels were related to the fact that 80 percent of the infections they saw were the Omicron variant, which causes more breakthrough cases.that has been a concern and no sign that the vaccine causes more high fevers than other routine vaccines.It depends on a few factors.
so that they can compare the two and possibly release them at the same time, like they did with the adult vaccine. That would obviously also take longer.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Read more »
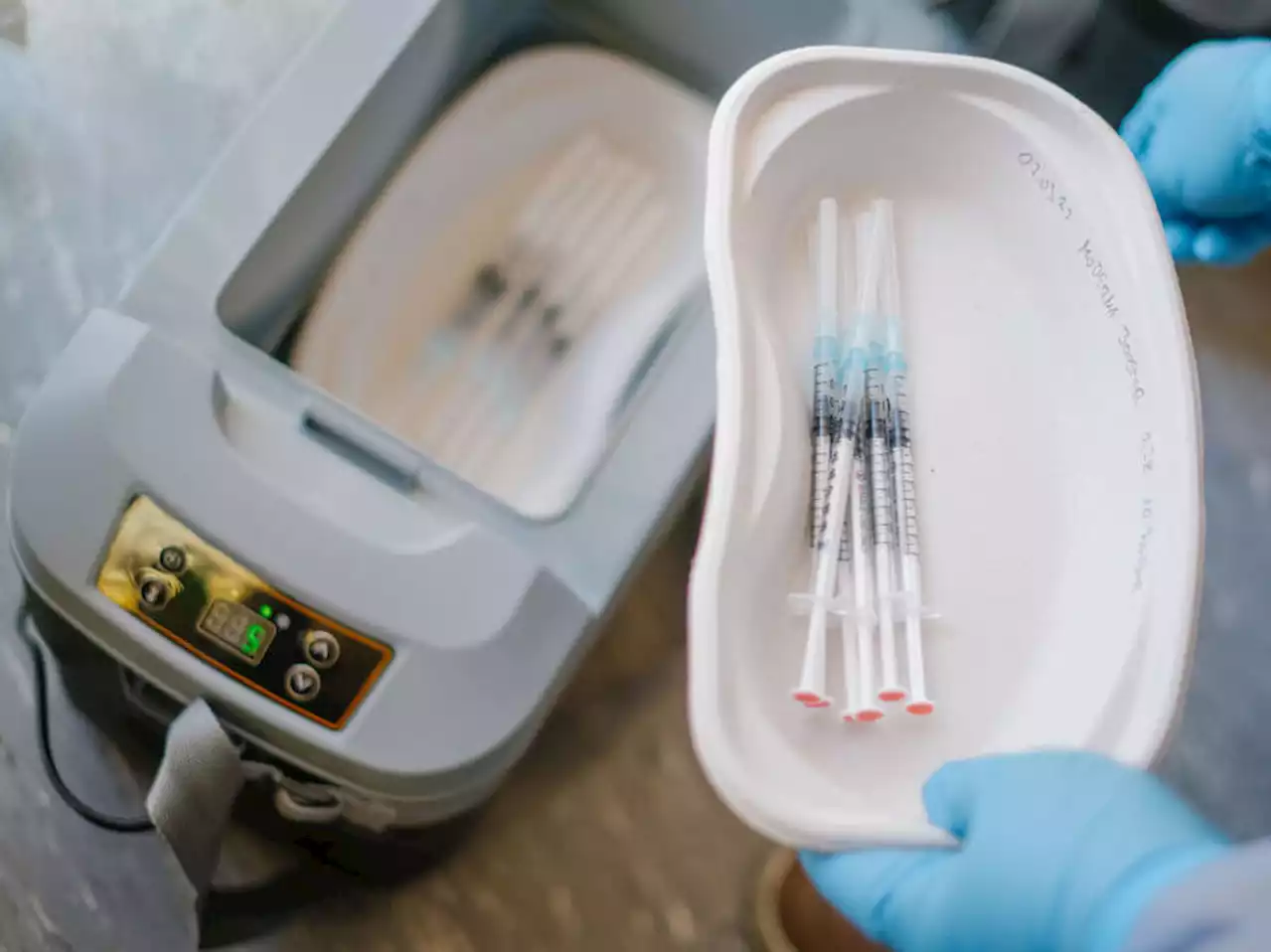 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Read more »
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Read more »
 Moderna Asks FDA to Authorize Covid Vaccine for Kids Under 6 Years OldThe FDA has promised to move quickly to authorize shots for infants, toddlers and preschoolers once the vaccine makers submit complete applications.
Moderna Asks FDA to Authorize Covid Vaccine for Kids Under 6 Years OldThe FDA has promised to move quickly to authorize shots for infants, toddlers and preschoolers once the vaccine makers submit complete applications.
Read more »
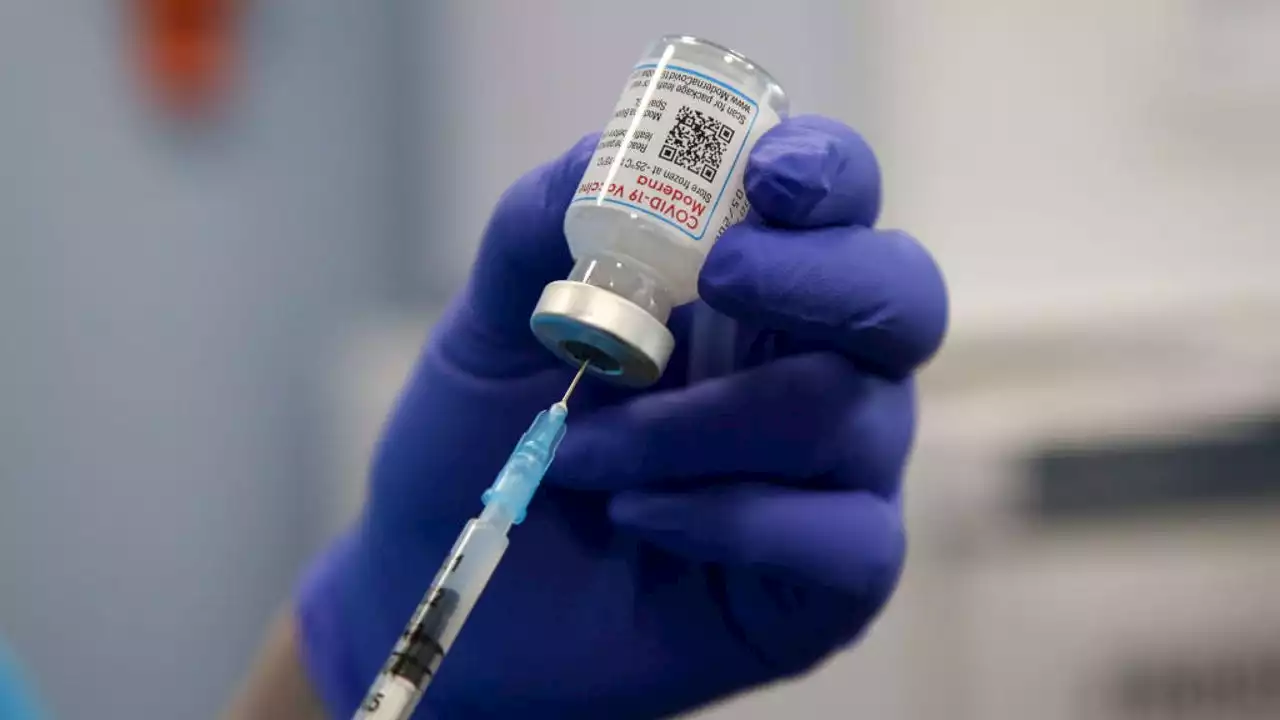 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Read more »
 Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Read more »
