CNN's Manu Raju asked House Speaker Mike Johnson if President Trump should defy court orders that he doesn't agree with after several judges have ruled against Trump. Johnson defended the work of Elon Musk and DOGE, saying they're uncovering waste that Congress has historically been unable to find.
We are currently undergoing maintenance on some services, which may temporarily affect access to subscription accounts and the E-edition. We apologize for any inconvenience and appreciate your patience as we work to resolve the issues.WATCH: First half highlights of Troy men's basketball game against Southern Miss
The countdown is on! Kansas City Chiefs and Philadelphia Eagles gear up for Super Bowl 59 in New OrleansWATCH: Dothan Mayor signs proclamation declaring February as Career and Technical Education MonthTrump administration halts $5 billion EV charging initiative started by BidenCNN's Manu Raju asked House Speaker Mike Johnson if President Trump should defy court orders that he doesn't agree with after several judges have ruled against Trump.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Johnson & Johnson Acquires Intra-Cellular Therapies for $14.6 BillionJohnson & Johnson unveils a major acquisition, purchasing Intra-Cellular Therapies for $14.6 billion to bolster its central nervous system disorder treatment portfolio. The deal focuses on Caplyta, a promising drug for schizophrenia and depression, with strong anticipated growth.
Johnson & Johnson Acquires Intra-Cellular Therapies for $14.6 BillionJohnson & Johnson unveils a major acquisition, purchasing Intra-Cellular Therapies for $14.6 billion to bolster its central nervous system disorder treatment portfolio. The deal focuses on Caplyta, a promising drug for schizophrenia and depression, with strong anticipated growth.
Read more »
 Johnson & Johnson to Acquire Intra-Cellular Therapies for $14.6 BillionJohnson & Johnson (J&J) is acquiring Intra-Cellular Therapies for over $14 billion, marking a significant investment in the treatment of central nervous system disorders. The deal includes a premium payment of $132 per share for Intra-Cellular, granting J&J ownership of Caplyta, a promising drug for schizophrenia and bipolar depression. J&J intends to leverage Intra-Cellular's pipeline, which includes potential treatments for anxiety, psychosis, and Alzheimer's-related agitation, to bolster its presence in this growing market.
Johnson & Johnson to Acquire Intra-Cellular Therapies for $14.6 BillionJohnson & Johnson (J&J) is acquiring Intra-Cellular Therapies for over $14 billion, marking a significant investment in the treatment of central nervous system disorders. The deal includes a premium payment of $132 per share for Intra-Cellular, granting J&J ownership of Caplyta, a promising drug for schizophrenia and bipolar depression. J&J intends to leverage Intra-Cellular's pipeline, which includes potential treatments for anxiety, psychosis, and Alzheimer's-related agitation, to bolster its presence in this growing market.
Read more »
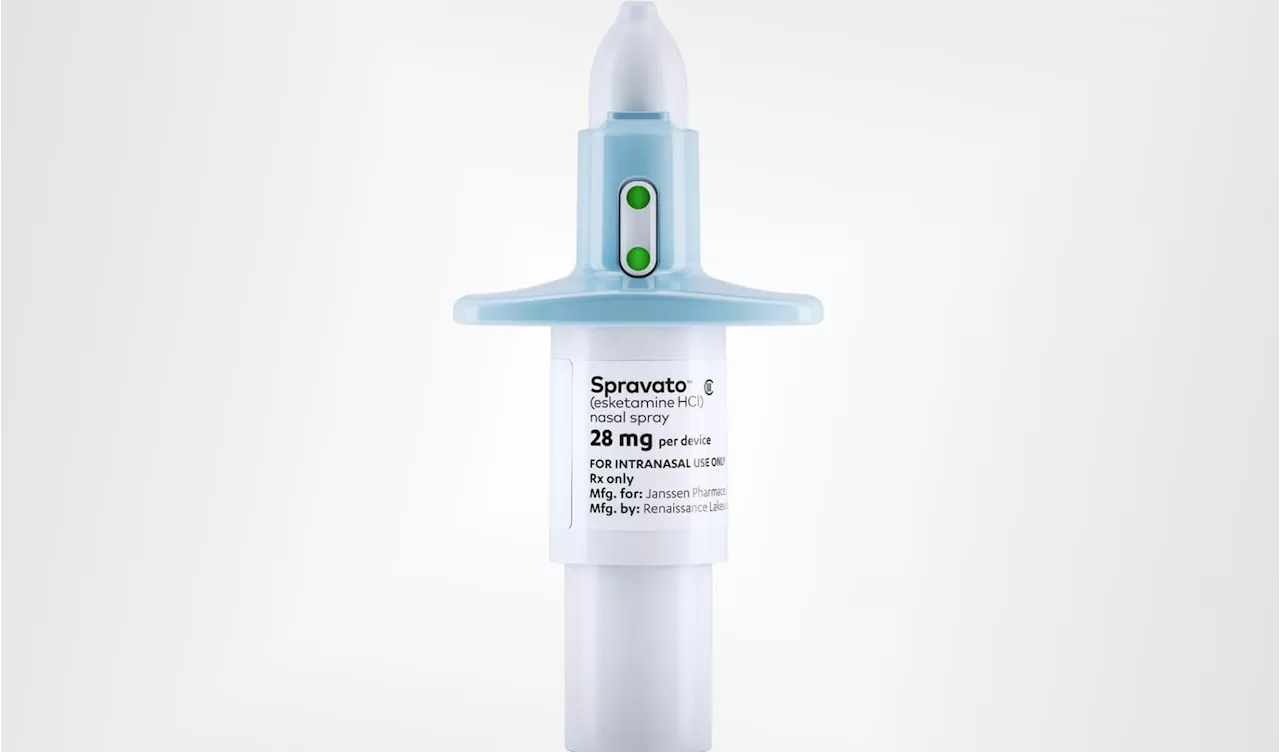 FDA approves Johnson & Johnson's nasal spray for depression as standalone treatmentSpravato is now the first-ever standalone therapy for treatment-resistant depression, and is on its way to becoming a blockbuster product.
FDA approves Johnson & Johnson's nasal spray for depression as standalone treatmentSpravato is now the first-ever standalone therapy for treatment-resistant depression, and is on its way to becoming a blockbuster product.
Read more »
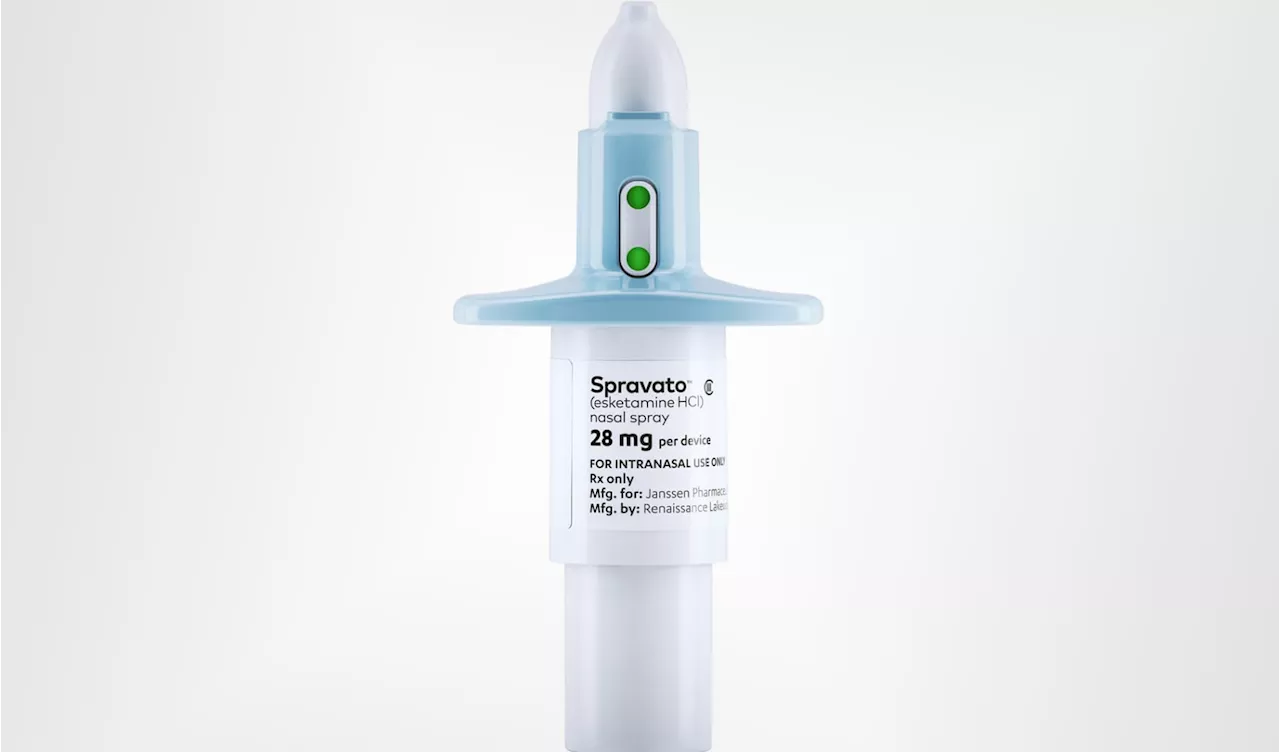 Johnson & Johnson's Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe Food and Drug Administration (FDA) has approved Johnson & Johnson's nasal spray, Spravato, as the first standalone therapy for treatment-resistant depression. This marks a significant advancement in treating individuals whose depression symptoms haven't responded to at least two standard treatments. Spravato, previously approved for use alongside oral antidepressants, has shown promising results in relieving symptoms rapidly and durably.
Johnson & Johnson's Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe Food and Drug Administration (FDA) has approved Johnson & Johnson's nasal spray, Spravato, as the first standalone therapy for treatment-resistant depression. This marks a significant advancement in treating individuals whose depression symptoms haven't responded to at least two standard treatments. Spravato, previously approved for use alongside oral antidepressants, has shown promising results in relieving symptoms rapidly and durably.
Read more »
 Johnson & Johnson's Nasal Spray Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe FDA approved Johnson & Johnson's Spravato nasal spray as the first-ever standalone therapy for treatment-resistant depression, offering new hope for millions suffering from this challenging condition.
Johnson & Johnson's Nasal Spray Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe FDA approved Johnson & Johnson's Spravato nasal spray as the first-ever standalone therapy for treatment-resistant depression, offering new hope for millions suffering from this challenging condition.
Read more »
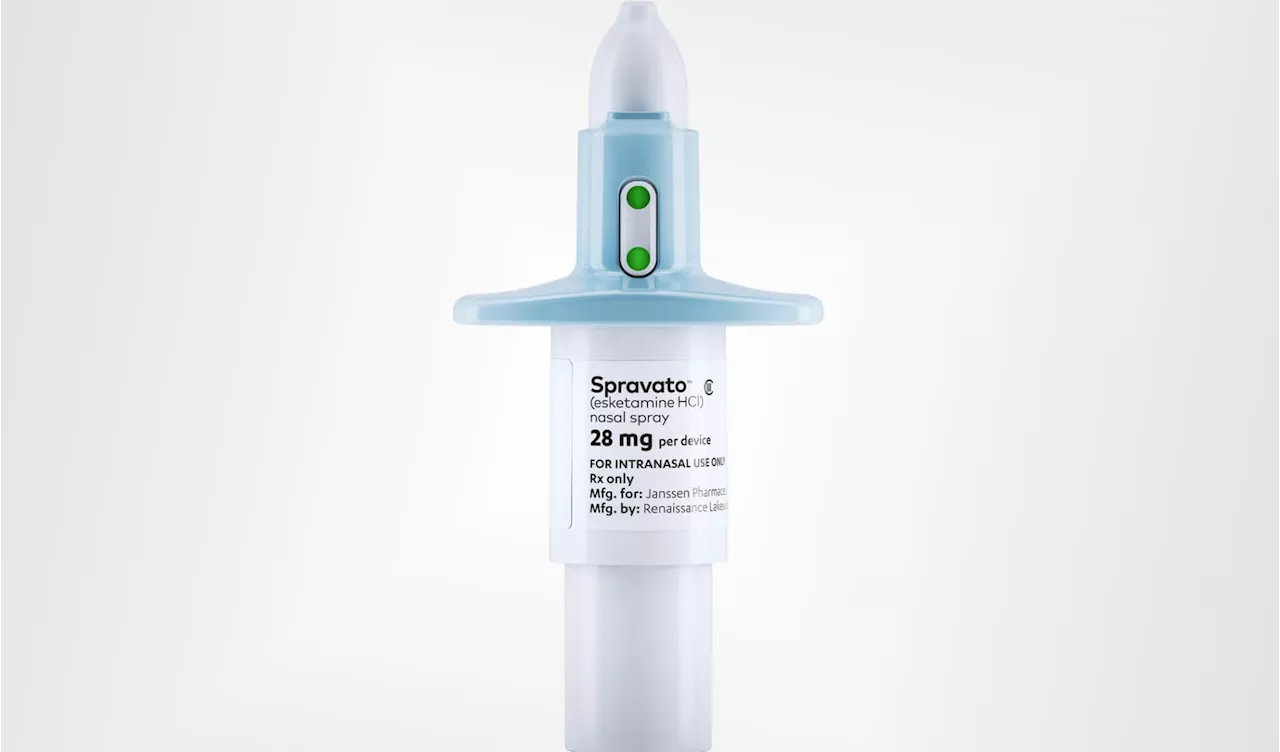 Johnson & Johnson's Spravato Approved as First Standalone Therapy for Treatment-Resistant DepressionThe FDA approves Spravato (esketamine) nasal spray as a standalone treatment for adults with treatment-resistant major depressive disorder.
Johnson & Johnson's Spravato Approved as First Standalone Therapy for Treatment-Resistant DepressionThe FDA approves Spravato (esketamine) nasal spray as a standalone treatment for adults with treatment-resistant major depressive disorder.
Read more »
