LIVE: White House COVID-19 response briefing; millions of vaccine shots ordered for youngest.
Up to about 20 million U.S. children under 5 would become eligible for vaccination if the government authorizes one or both shots.
And public health officials have been disappointed at how many older U.S. children, who have been eligible for shots for months, have yet to be vaccinated: Less than one-third of kids ages 5 to 11 have gotten the two recommended doses, according to government figures. Moderna has asked the FDA to authorize two shots for kids ages 6 months to 5 years, each containing a quarter of the dose given to adults.
An FDA advisory committee is scheduled to meet Tuesday and Wednesday to review data from the two companies. Officials say they expect a FDA decision shortly after that meeting.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 White House outlines COVID-19 aid for Americas ahead of Los Angeles summitThe summit is designed to tackle key regional issues but Mexico is sending a lower-level delegation, instead of its president, to protest Biden’s decision to exclude Cuba, Nicaragua and Venezuela.
White House outlines COVID-19 aid for Americas ahead of Los Angeles summitThe summit is designed to tackle key regional issues but Mexico is sending a lower-level delegation, instead of its president, to protest Biden’s decision to exclude Cuba, Nicaragua and Venezuela.
Read more »
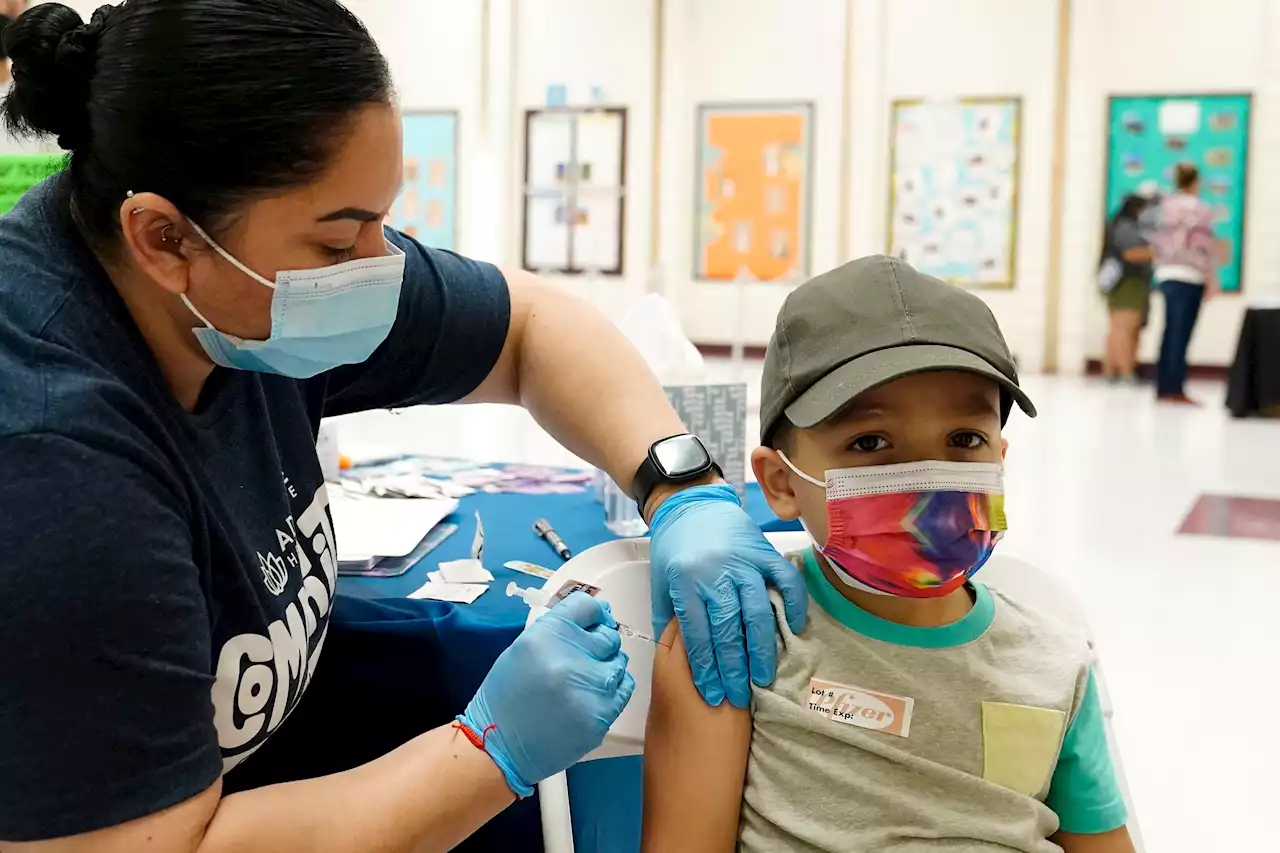 White House Lays Out Plan To Get COVID-19 Vaccines To Kids Under 5Vaccines for children younger than 5 could be available as soon as late June, according to senior Biden administration officials.
White House Lays Out Plan To Get COVID-19 Vaccines To Kids Under 5Vaccines for children younger than 5 could be available as soon as late June, according to senior Biden administration officials.
Read more »
 Coronavirus: White House unveils operational plan for COVID-19 vaccines for young kidsThe White House on Thursday unveiled an operational plan to make COVID-19 vaccines available to young children if the shots are approved.
Coronavirus: White House unveils operational plan for COVID-19 vaccines for young kidsThe White House on Thursday unveiled an operational plan to make COVID-19 vaccines available to young children if the shots are approved.
Read more »
 White House pressured to scrap COVID-19 testing requirement for travel to USThe Biden administration is facing pressure to end a COVID-19 travel restriction that tourism industry advocates say is hurting the economy and causing headaches for Americans and foreign nationals alike.
White House pressured to scrap COVID-19 testing requirement for travel to USThe Biden administration is facing pressure to end a COVID-19 travel restriction that tourism industry advocates say is hurting the economy and causing headaches for Americans and foreign nationals alike.
Read more »
 COVID-19 and Kids - A Live AMACOVID-19 has challenged parents to manage their children’s health in a setting of unprecedented risk. In this live virtual event, KPCC/LAist health reporter Jackie Fortiér will delve into the effects of COVID-19 on kids and the upcoming vaccination for kids under five years old.
COVID-19 and Kids - A Live AMACOVID-19 has challenged parents to manage their children’s health in a setting of unprecedented risk. In this live virtual event, KPCC/LAist health reporter Jackie Fortiér will delve into the effects of COVID-19 on kids and the upcoming vaccination for kids under five years old.
Read more »
 FDA advisers meeting on Novavax, a latecomer in COVID-19 vaccine raceA federal advisory committee will vote on whether regulators should authorize a COVID-19 vaccine made by Novavax. From The New York Times.
FDA advisers meeting on Novavax, a latecomer in COVID-19 vaccine raceA federal advisory committee will vote on whether regulators should authorize a COVID-19 vaccine made by Novavax. From The New York Times.
Read more »
