Johnson & Johnson announced a breakthrough in mental health treatment with FDA approval of Spravato, a nasal spray, for standalone treatment of major depressive disorder (MDD), post-traumatic stress disorder (PTSD), and similar conditions. Previously available only in combination with oral antidepressants, Spravato offers hope for treatment-resistant depression. While the exact mechanism remains unclear, it acts on the NMDA receptor and influences the neurotransmitter glutamate. The FDA approved Spravato based on a study showing significant improvement in depression scores in patients who received the medication compared to a placebo.
Johnson & Johnson has announced a major breakthrough in mental health treatment. The Food and Drug Administration has approved Spravato , a novel nasal spray, for the standalone treatment of major depressive disorder , post-traumatic stress disorder , and other similar psychiatric conditions.This approval marks a significant milestone as the medication was previously only available when combined with oral antidepressants.
How Spravato worksSpravato differs from traditional antidepressants in its mechanism of action. Like many antidepressants, it acts as a non-selective, non-competitive antagonist of the N-methyl-D-aspartate receptor. However, it also works through an alternate brain pathway that influences the neurotransmitter glutamate. An imbalance in glutamate levels has been linked to symptoms like exhaustion, difficulty concentrating, and depression.
MENTAL HEALTH DEPRESSION PTSD SPRAVATO Ketamine
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
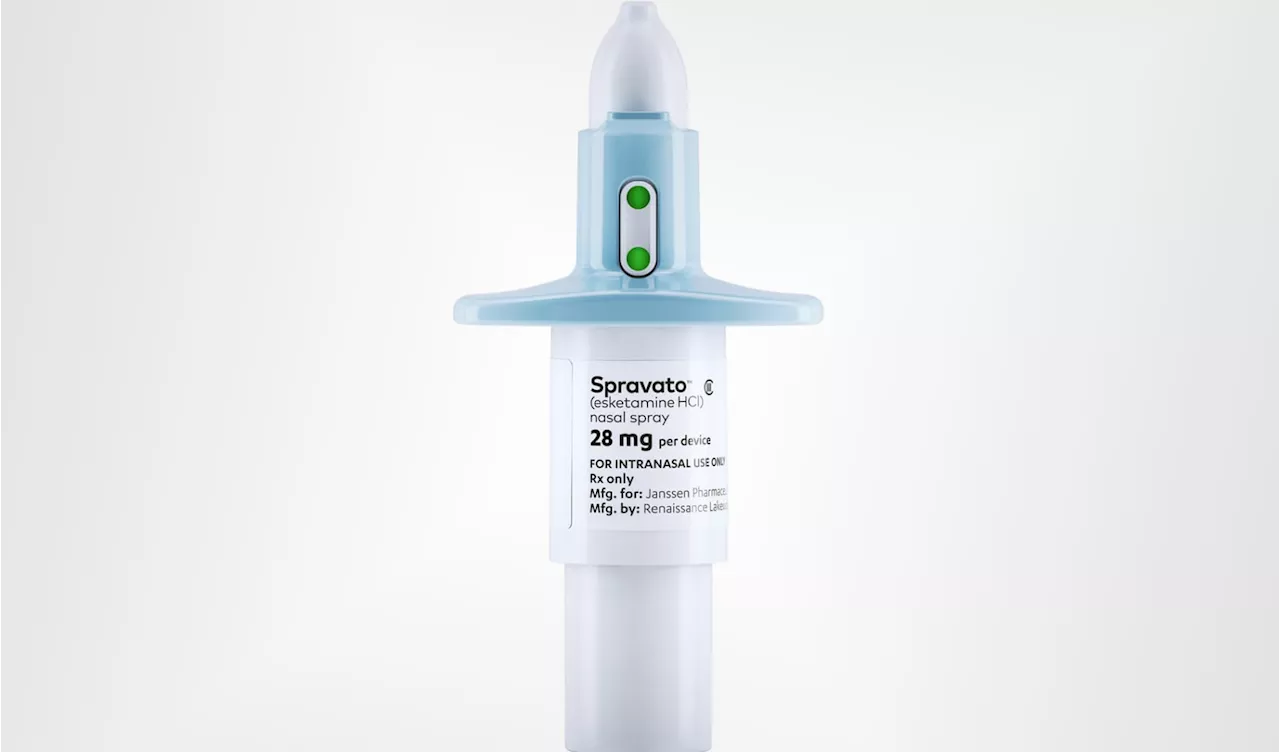 Johnson & Johnson's Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe Food and Drug Administration (FDA) has approved Johnson & Johnson's nasal spray, Spravato, as the first standalone therapy for treatment-resistant depression. This marks a significant advancement in treating individuals whose depression symptoms haven't responded to at least two standard treatments. Spravato, previously approved for use alongside oral antidepressants, has shown promising results in relieving symptoms rapidly and durably.
Johnson & Johnson's Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe Food and Drug Administration (FDA) has approved Johnson & Johnson's nasal spray, Spravato, as the first standalone therapy for treatment-resistant depression. This marks a significant advancement in treating individuals whose depression symptoms haven't responded to at least two standard treatments. Spravato, previously approved for use alongside oral antidepressants, has shown promising results in relieving symptoms rapidly and durably.
Read more »
 Johnson & Johnson's Nasal Spray Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe FDA approved Johnson & Johnson's Spravato nasal spray as the first-ever standalone therapy for treatment-resistant depression, offering new hope for millions suffering from this challenging condition.
Johnson & Johnson's Nasal Spray Spravato Approved as Standalone Treatment for Treatment-Resistant DepressionThe FDA approved Johnson & Johnson's Spravato nasal spray as the first-ever standalone therapy for treatment-resistant depression, offering new hope for millions suffering from this challenging condition.
Read more »
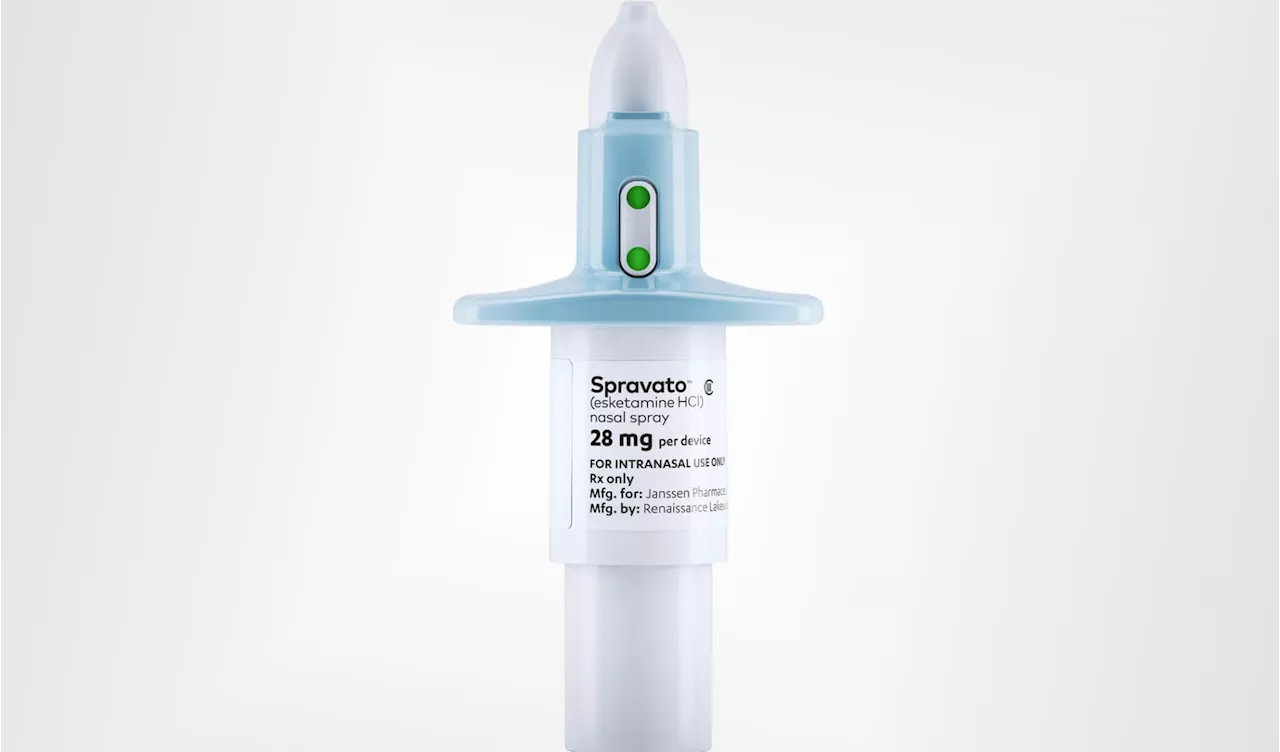 Johnson & Johnson's Spravato Approved as First Standalone Therapy for Treatment-Resistant DepressionThe FDA approves Spravato (esketamine) nasal spray as a standalone treatment for adults with treatment-resistant major depressive disorder.
Johnson & Johnson's Spravato Approved as First Standalone Therapy for Treatment-Resistant DepressionThe FDA approves Spravato (esketamine) nasal spray as a standalone treatment for adults with treatment-resistant major depressive disorder.
Read more »
 FDA Approves Spravato as Standalone Treatment for DepressionThe FDA has expanded the approval of Spravato, a nasal spray antidepressant derived from ketamine, to allow its use as a standalone treatment for depression.
FDA Approves Spravato as Standalone Treatment for DepressionThe FDA has expanded the approval of Spravato, a nasal spray antidepressant derived from ketamine, to allow its use as a standalone treatment for depression.
Read more »
 FDA allows standalone use of nasal spray antidepressant Spravato (esketamine)The FDA says esketamine, an antidepressant derived from the anesthetic and party drug ketamine, can now be prescribed on its own. It was approved in 2019 to treat severe depression.
FDA allows standalone use of nasal spray antidepressant Spravato (esketamine)The FDA says esketamine, an antidepressant derived from the anesthetic and party drug ketamine, can now be prescribed on its own. It was approved in 2019 to treat severe depression.
Read more »
 FDA Approves Johnson & Johnson's Ketamine Nasal Spray for Severe DepressionThe Food and Drug Administration (FDA) has approved Spravato, a ketamine-derived nasal spray developed by Johnson & Johnson, as a groundbreaking treatment for severe depression. This marks the first standalone therapy specifically for adults with major depressive disorder who haven't responded to traditional antidepressants.
FDA Approves Johnson & Johnson's Ketamine Nasal Spray for Severe DepressionThe Food and Drug Administration (FDA) has approved Spravato, a ketamine-derived nasal spray developed by Johnson & Johnson, as a groundbreaking treatment for severe depression. This marks the first standalone therapy specifically for adults with major depressive disorder who haven't responded to traditional antidepressants.
Read more »
