Trump officials 'deliberately and repeatedly' pressured the FDA to authorize unproven and potentially dangerous Covid-19 treatments while working to derail the agency's vaccine guidance ahead of the 2020 presidential election, a new House report reveals.
documenting what the panel calls the Trump administration's"rampant political interference with the federal public health response" to a pandemic that has now killed more than 1,040,000 people in the United States.
Former FDA Commissioner Dr. Stephen Hahn told the committee that Peter Navarro, who headed the White House Office of Trade and Manufacturing Policy,"exerted inappropriate pressure" on the FDA to renew the emergency use authorization for the anti-malarial drug hydroxychloroquine as a Covid-19 treatment, even after it was shown to be ineffective and possibly dangerous.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Trump staffers pushed unproven COVID treatment at FDA: PanelOfficials in the Trump White House tried to pressure U.S. health experts into reauthorizing a discredited COVID-19 treatment.
Trump staffers pushed unproven COVID treatment at FDA: PanelOfficials in the Trump White House tried to pressure U.S. health experts into reauthorizing a discredited COVID-19 treatment.
Read more »
 Trump Staffers Pushed Unproven COVID Treatment at FDA, House Report FindsA special House panel looking into the government’s coronavirus response says the Trump White House tried to pressure U.S. health experts into reauthorizing the drug hydroxychloroquine that had been discredited as a COVID-19 treatment.
Trump Staffers Pushed Unproven COVID Treatment at FDA, House Report FindsA special House panel looking into the government’s coronavirus response says the Trump White House tried to pressure U.S. health experts into reauthorizing the drug hydroxychloroquine that had been discredited as a COVID-19 treatment.
Read more »
 Panel: Trump staffers pushed unproven COVID treatment at FDAA special House panel looking into the government's coronavirus response says the Trump White House tried to pressure U.S. health experts into reauthorizing the drug hydroxychloroquine that had been discredited as a COVID-19 treatment.
Panel: Trump staffers pushed unproven COVID treatment at FDAA special House panel looking into the government's coronavirus response says the Trump White House tried to pressure U.S. health experts into reauthorizing the drug hydroxychloroquine that had been discredited as a COVID-19 treatment.
Read more »
 Trump White House exerted pressure on FDA for Covid-19 emergency use authorizations, House report findsThe Trump administration pressured the FDA to authorize unproven treatments for Covid-19 and the first Covid-19 vaccines on an accelerated timeline, according to a House report released Wednesday.
Trump White House exerted pressure on FDA for Covid-19 emergency use authorizations, House report findsThe Trump administration pressured the FDA to authorize unproven treatments for Covid-19 and the first Covid-19 vaccines on an accelerated timeline, according to a House report released Wednesday.
Read more »
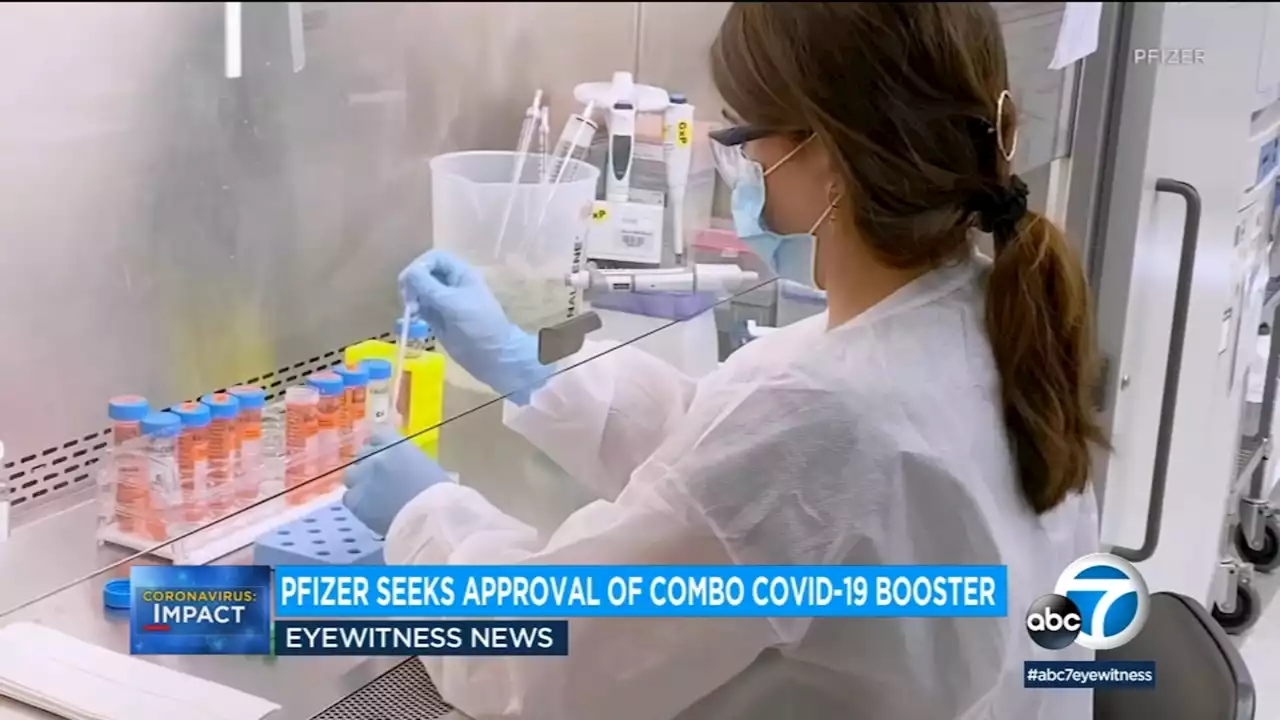 Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Read more »
 Pfizer-BioNTech submits new COVID vaccine booster targeting BA.5 to the FDA for authorizationPfizer and German partner BioNTech have submitted their new COVID-19 booster – that targets the omicron subvariant BA.5 – to the FDA for emergency use authorization.
Pfizer-BioNTech submits new COVID vaccine booster targeting BA.5 to the FDA for authorizationPfizer and German partner BioNTech have submitted their new COVID-19 booster – that targets the omicron subvariant BA.5 – to the FDA for emergency use authorization.
Read more »
