People who got a fourth shot of the Pfizer/BioNTech vaccine had a fivefold increase in antibodies one week later, according to data from Israel
a fourth dose of the Covid-19 vaccine
from Pfizer Inc. and BioNTech SE will provide safe and effective protection against infection and severe illness for those with waning immunity. There was a fivefold increase in antibodies of individuals one week after they got their fourth shot, according to data from Israel’s Sheba Medical Center, which provided the dose to 154 medical workers of various ages. The personnel who took part in the trial had taken a third shot by Aug. 20 and their tests showed they lacked sufficient antibodies for good protection.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
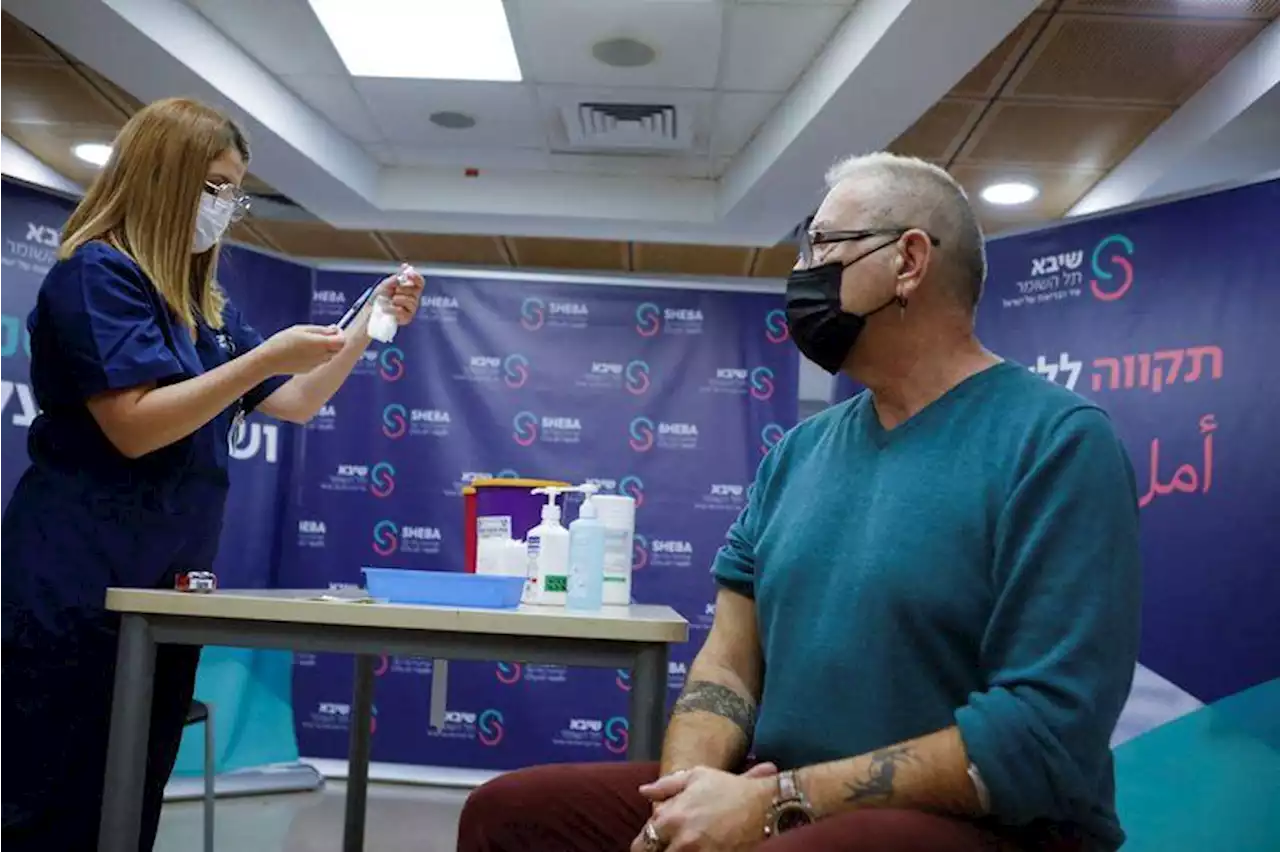 Israel to offer fourth COVID vaccine shot to over 60s, medical staffJERUSALEM (Reuters) -Prime Minister Naftali Bennett said on Sunday that Israel would offer a fourth dose of COVID-19 vaccine to people over 60 and to medical staff as it faces a surge in Omicron variant infections. Israel last week approved a fourth dose of the vaccine developed by Pfizer and BioNTech, a second booster, for people who are immune-compromised and the elderly living in care homes. 'We now have a new layer of defence,' Bennett said in a televised news conference, adding that Israel's top government medical official, whose approval is needed to expand the booster campaign, had signed off on the latest move.
Israel to offer fourth COVID vaccine shot to over 60s, medical staffJERUSALEM (Reuters) -Prime Minister Naftali Bennett said on Sunday that Israel would offer a fourth dose of COVID-19 vaccine to people over 60 and to medical staff as it faces a surge in Omicron variant infections. Israel last week approved a fourth dose of the vaccine developed by Pfizer and BioNTech, a second booster, for people who are immune-compromised and the elderly living in care homes. 'We now have a new layer of defence,' Bennett said in a televised news conference, adding that Israel's top government medical official, whose approval is needed to expand the booster campaign, had signed off on the latest move.
Read more »
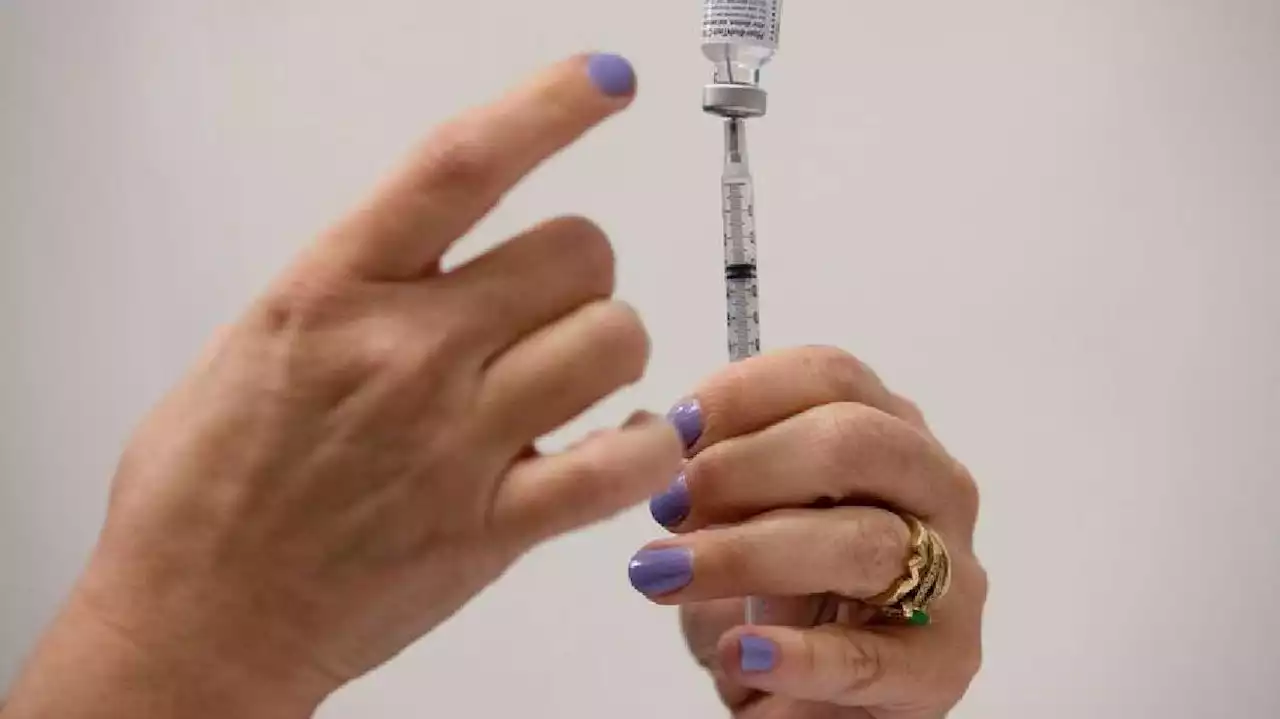 CDC recommends 5-month gap for Pfizer COVID-19 booster doseThe U.S. Centers for Disease Control and Prevention on Tuesday recommended shortening the interval between Pfizer-BioNTech's second COVID-19 vaccine dose and the booster shot to five months from six.
CDC recommends 5-month gap for Pfizer COVID-19 booster doseThe U.S. Centers for Disease Control and Prevention on Tuesday recommended shortening the interval between Pfizer-BioNTech's second COVID-19 vaccine dose and the booster shot to five months from six.
Read more »
 Pediatrician answers your questions on Pfizer Covid-19 booster for ages 12-15The FDA gave emergency use authorization for booster doses of Pfizer/BioNTech's Covid-19 vaccine for children ages 12 to 15 on Monday. A pediatrician answers parents' questions about the shots.
Pediatrician answers your questions on Pfizer Covid-19 booster for ages 12-15The FDA gave emergency use authorization for booster doses of Pfizer/BioNTech's Covid-19 vaccine for children ages 12 to 15 on Monday. A pediatrician answers parents' questions about the shots.
Read more »
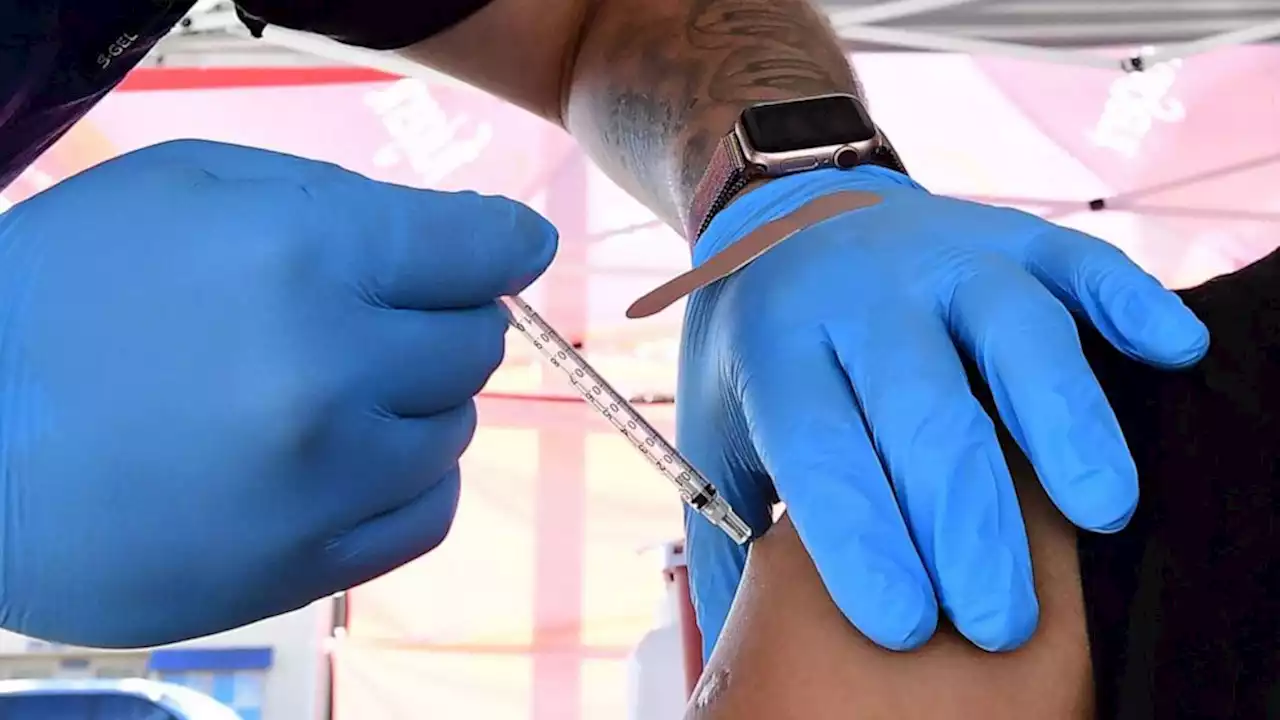 FDA authorizes Pfizer COVID-19 boosters for 12- to 15-year oldsThe FDA on Monday authorized Pfizer COVID-19 boosters for 12- to 15-year olds amid the omicron surge as children head back to school.
FDA authorizes Pfizer COVID-19 boosters for 12- to 15-year oldsThe FDA on Monday authorized Pfizer COVID-19 boosters for 12- to 15-year olds amid the omicron surge as children head back to school.
Read more »
 FDA Backs Pfizer Booster for 12- to 15-Year-OldsThe agency also took two related actions in the revised emergency use authorization for the Pfizer/BioNTech vaccine.
FDA Backs Pfizer Booster for 12- to 15-Year-OldsThe agency also took two related actions in the revised emergency use authorization for the Pfizer/BioNTech vaccine.
Read more »
