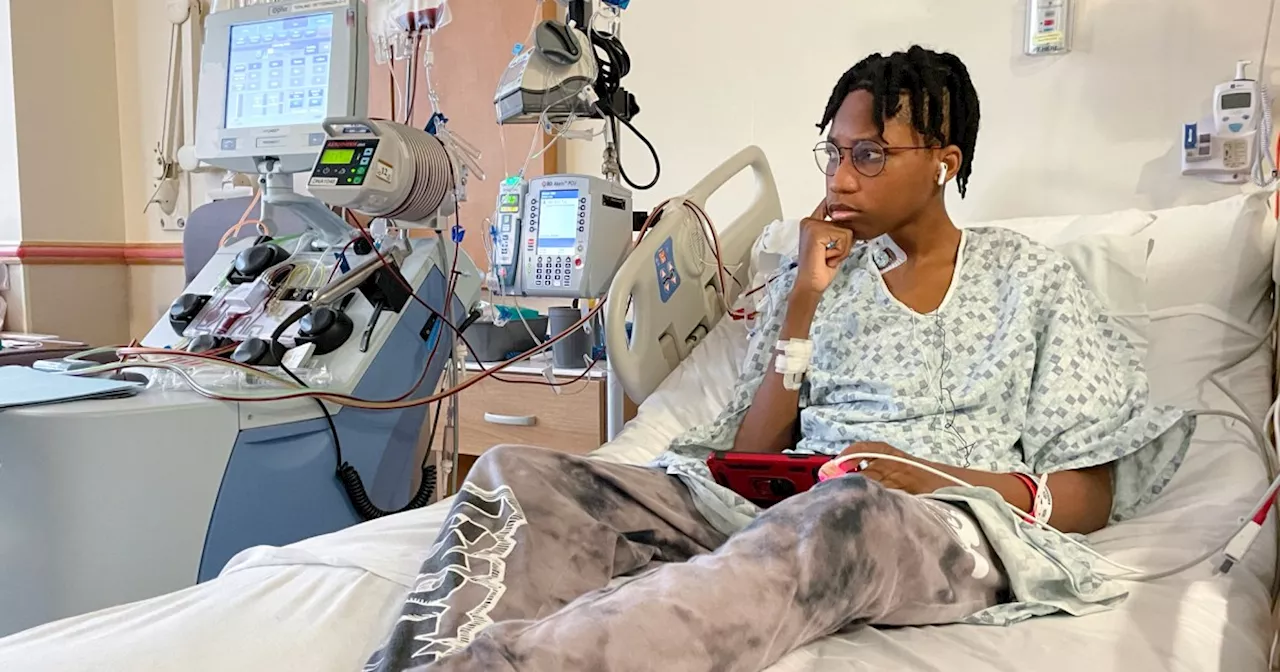
For Wedam Minyila, a 19-year-old with sickle cell disease, a recent hospital visit marked a potential turning point. He was among the first patients worldwide to undergo commercial treatment for the genetic condition, which could lead to a cure. Though still in its early stages, the gene therapy holds immense promise for the over 100,000 people in the U.S. living with sickle cell disease, offering the possibility of a life free from excruciating pain and debilitating complications.
WASHINGTON — For Wedam Minyila, hospital rooms have always meant blinding pain. “Like someone is jamming a knife in me,” he said. But for a brief moment on a recent December morning, Wedam, 19, who has sickle cell disease, allowed himself to believe what his doctors had been telling him for months: This visit could be the first step to a cure.
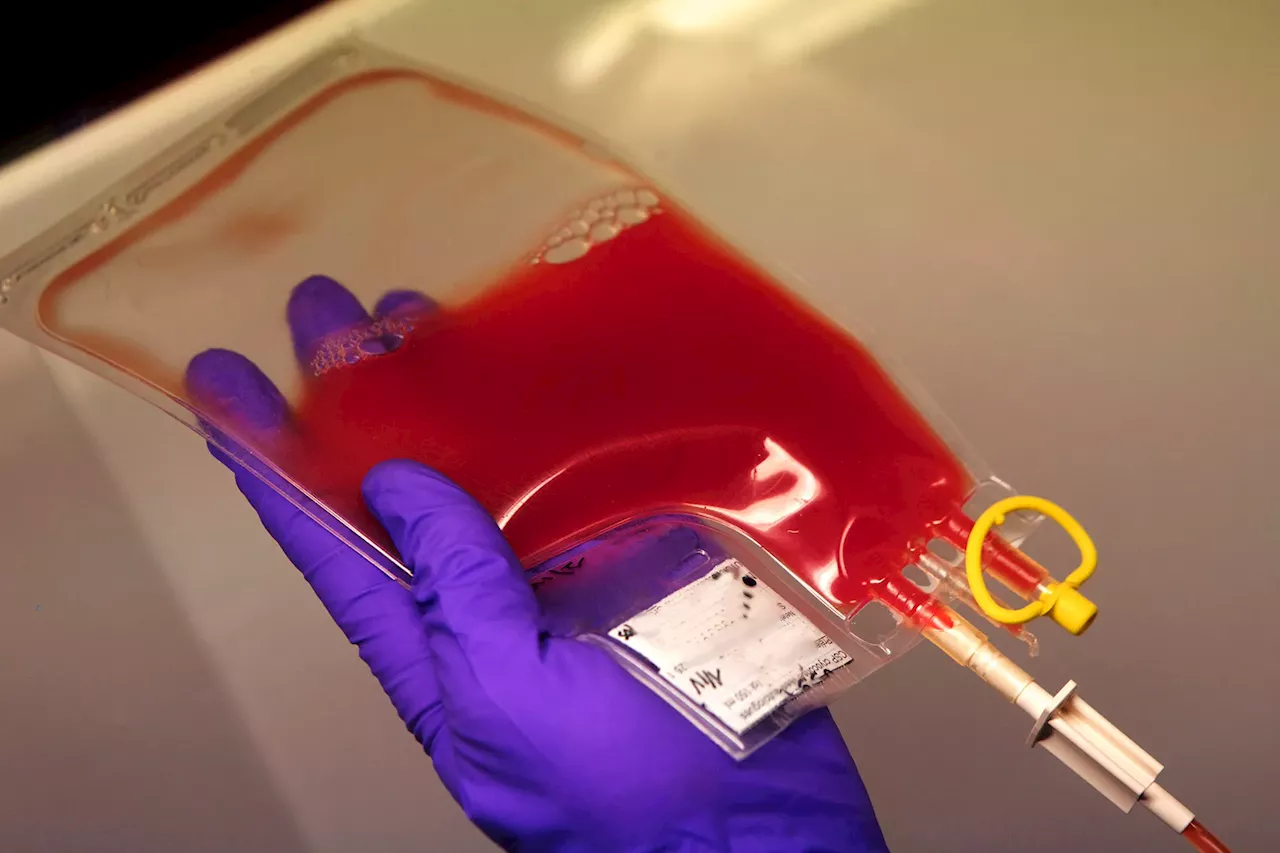
” “Remember, this is a very high-risk treatment requiring high-dose chemotherapy with potential for complications,” he said. “So it’s not something that we would want to ramp up too rapidly.” The monthslong treatment Casgevy from Vertex Pharmaceuticals comes with a list price of $2.3 million. Bluebird Bio’s Lyfgenia is listed at $3.1 million. Neither includes the cost of care to stay in the hospital or for chemotherapy.
GENE THERAPY SICKLE CELL DISEASE MEDICAL BREAKTHROUGH CRISPR TREATMENT
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 First Patients Receive Gene-Editing Treatment for Sickle CellCasgevy, the first CRISPR-based medicine, is being rolled out slowly due to manufacturing and insurance hurdles.
First Patients Receive Gene-Editing Treatment for Sickle CellCasgevy, the first CRISPR-based medicine, is being rolled out slowly due to manufacturing and insurance hurdles.
Read more »
 Casgevy: A Promise of Relief for Sickle Cell Patients Faces Slow RolloutA groundbreaking treatment using CRISPR gene editing, Casgevy, offers hope for patients with sickle cell disease. However, its commercialization faces hurdles, resulting in a limited number of patients receiving the therapy.
Casgevy: A Promise of Relief for Sickle Cell Patients Faces Slow RolloutA groundbreaking treatment using CRISPR gene editing, Casgevy, offers hope for patients with sickle cell disease. However, its commercialization faces hurdles, resulting in a limited number of patients receiving the therapy.
Read more »
 Archer: Abu Dhabi First City With Commercial EVTOL Air Taxis In 2025If we really want to get across the city fast, we need to look up – into the sky. Abu Dhabi will be the first city to have commercial eVTOL air taxis flights, in 2025.
Archer: Abu Dhabi First City With Commercial EVTOL Air Taxis In 2025If we really want to get across the city fast, we need to look up – into the sky. Abu Dhabi will be the first city to have commercial eVTOL air taxis flights, in 2025.
Read more »
 FDA Approves First Stem Cell Therapy for Life-Threatening Condition After Bone Marrow TransplantsThe U.S. Food and Drug Administration (FDA) has approved Remestemcel-L (Ryoncil), the first stem cell therapy for severe acute graft-versus-host disease (SR-aGVHD), a serious complication that can occur after bone marrow transplantation. SR-aGVHD affects about 25% of the 1,500 children in the U.S. who undergo bone marrow transplants annually. Ryoncil is derived from donor bone marrow stem cells and helps control the immune system's response by reducing inflammation and boosting the body's natural anti-inflammatory processes. The drug is also the first FDA-approved treatment for children ages 2 months and older with SR-aGVHD.
FDA Approves First Stem Cell Therapy for Life-Threatening Condition After Bone Marrow TransplantsThe U.S. Food and Drug Administration (FDA) has approved Remestemcel-L (Ryoncil), the first stem cell therapy for severe acute graft-versus-host disease (SR-aGVHD), a serious complication that can occur after bone marrow transplantation. SR-aGVHD affects about 25% of the 1,500 children in the U.S. who undergo bone marrow transplants annually. Ryoncil is derived from donor bone marrow stem cells and helps control the immune system's response by reducing inflammation and boosting the body's natural anti-inflammatory processes. The drug is also the first FDA-approved treatment for children ages 2 months and older with SR-aGVHD.
Read more »
 Vast Unveils Final Design for Haven-1, the World’s First Commercial Space StationVast Space is planning to deploy Haven-1, a single-module space station, in 2024, aiming to become the first commercial station in orbit. A successful deployment, including crewed missions, could secure Vast Space's bid for NASA's Commercial Low-Earth Orbit Destination (CLD) Phase II program. This would pave the way for the Haven-2 facility, a more complex, multi-module station planned for deployment in 2028.
Vast Unveils Final Design for Haven-1, the World’s First Commercial Space StationVast Space is planning to deploy Haven-1, a single-module space station, in 2024, aiming to become the first commercial station in orbit. A successful deployment, including crewed missions, could secure Vast Space's bid for NASA's Commercial Low-Earth Orbit Destination (CLD) Phase II program. This would pave the way for the Haven-2 facility, a more complex, multi-module station planned for deployment in 2028.
Read more »
 China’s first commercial heavy-water reactor for isotopes begins productionQinshan Nuclear Power Base plant in China has opened its doors, producing medical isotopes using online irradiation.
China’s first commercial heavy-water reactor for isotopes begins productionQinshan Nuclear Power Base plant in China has opened its doors, producing medical isotopes using online irradiation.
Read more »