Federal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD.
6 years ago, the roof of a Jacksonville strip mall partially collapsed. Now that it’s finally demolished, what’s next?FILE- A sign is displayed for the Food and Drug Administration offices in Silver Spring, Md., on Dec. 10, 2020. Drugmaker Lykos Therapeutics said the FDA notified the company that its drug “could not be approved based on data submitted to date,” and requested an additional late-stage study. Such studies generally takes several years and millions of dollars to conduct.
“The FDA request for another study is deeply disappointing," Lykos CEO Amy Emerson said Friday in a statement. “Our heart breaks for the millions of military veterans, first responders, victims of sexual and domestic abuse and countless others suffering from PTSD who may now face more years without access to new treatment options.”
Antidepressants are now the only FDA-approved drugs for PTSD, which is closely linked to depression, anxiety and suicidal thinking and is more prevalent among women and veterans., who say the lack of treatments options for the condition has contributed to higher rates of suicide among military personnel. Last month, veterans supporting psychedelic therapy rallied on Capitol Hill in support of the drug.
The idea of using psychedelics to enhance psychotherapy is not new. A handful of therapists in California used MDMA during the 1970s and 1980s — when it was still legal — to facilitate couples therapy sessions. MAPS was founded in 1986 to oppose a federal decision placing MDMA in the same ultra-restrictive drug category as heroin, LSD and other illegal psychedelics.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA won't approve psychedelic MDMA for PTSD, calling for additional studyFederal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD. Drugmaker Lykos Therapeutics announced the FDA's decision on Friday.
FDA won't approve psychedelic MDMA for PTSD, calling for additional studyFederal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD. Drugmaker Lykos Therapeutics announced the FDA's decision on Friday.
Read more »
 FDA won't approve psychedelic MDMA for PTSD, calling for additional studyFederal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD.
FDA won't approve psychedelic MDMA for PTSD, calling for additional studyFederal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD.
Read more »
 FDA won't approve psychedelic MDMA for PTSD, calling for additional studyFederal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD.
FDA won't approve psychedelic MDMA for PTSD, calling for additional studyFederal health officials have declined to approve the psychedelic drug MDMA as a therapy for PTSD.
Read more »
 FDA rejects psychedelic MDMA-assisted therapy for PTSDBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA rejects psychedelic MDMA-assisted therapy for PTSDBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Read more »
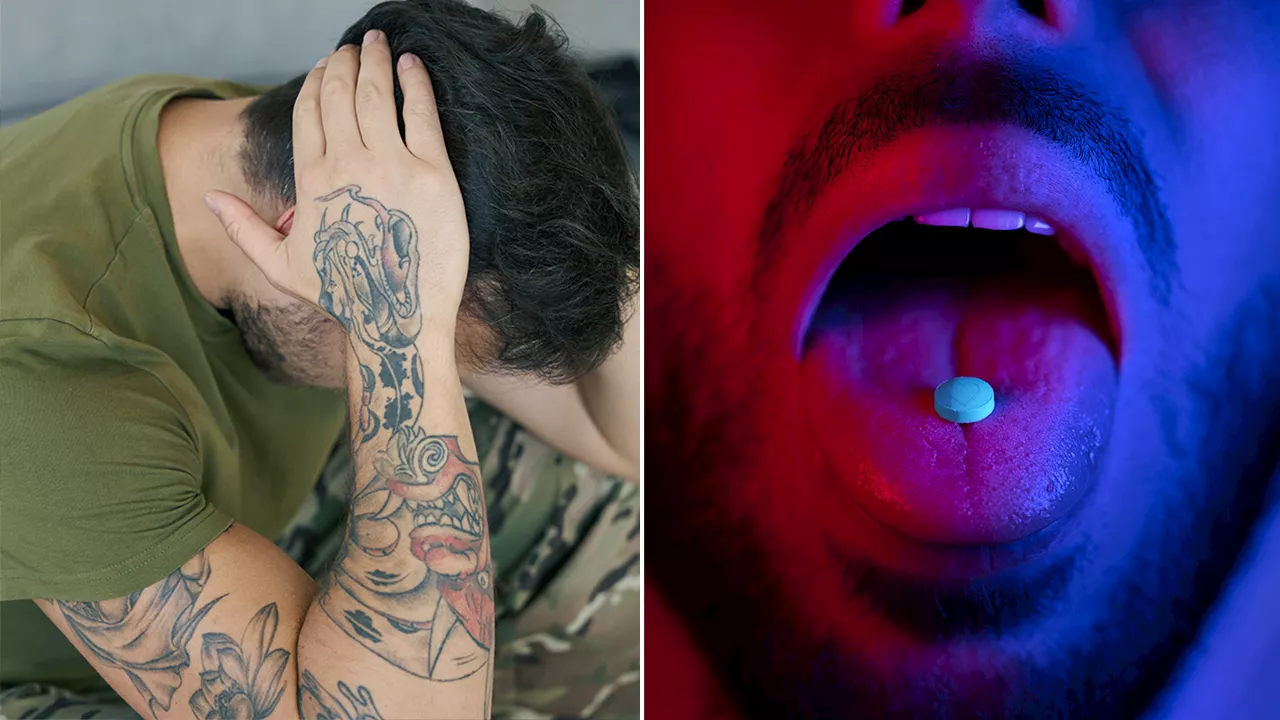 Veterans advocate for MDMA, also known as ecstasy, to be used in therapy to treat PTSDThe FDA's decision Aug. 11 could mark a major shift in PTSD treatment for veterans. MDMA-AT, more commonly known as ecstasy or molly, shows promising results in therapy.
Veterans advocate for MDMA, also known as ecstasy, to be used in therapy to treat PTSDThe FDA's decision Aug. 11 could mark a major shift in PTSD treatment for veterans. MDMA-AT, more commonly known as ecstasy or molly, shows promising results in therapy.
Read more »
 Bipartisan House and Senate group pushes Biden administration for MDMA treatment of PTSDPolitical News and Conservative Analysis About Congress, the President, and the Federal Government
Bipartisan House and Senate group pushes Biden administration for MDMA treatment of PTSDPolitical News and Conservative Analysis About Congress, the President, and the Federal Government
Read more »
