THOUGHTS? 🚬 FDA chief says agency will release details of long-awaited plan to ban menthol in cigarettes, cigars -
Food and Drug Administration Commissioner Robert Califf previewed the announcement in congressional testimony, saying the proposal would reduce disease and death by helping current smokers quit and stopping younger people from starting.
The FDA has attempted several times to get rid of menthol but faced pushback from Big Tobacco, members of Congress and competing political interests under both Democratic and Republican administrations. The FDA will also seek to ban menthol and dozens of over sweet and fruity flavors from small cigars, which are increasingly popular with young people, especially Black teens.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA to issue plan banning menthol in cigarettes, cigarsMenthol accounts for more than a third of cigarettes sold in the U.S., and the flavor is overwhelmingly favored by Black smokers and young people.
FDA to issue plan banning menthol in cigarettes, cigarsMenthol accounts for more than a third of cigarettes sold in the U.S., and the flavor is overwhelmingly favored by Black smokers and young people.
Read more »
 FDA Announces Recall of Popular Blood Pressure Medication Due to Potential Cancer RiskIt’s time to double-check your medicine cabinets.
FDA Announces Recall of Popular Blood Pressure Medication Due to Potential Cancer RiskIt’s time to double-check your medicine cabinets.
Read more »
 FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
Read more »
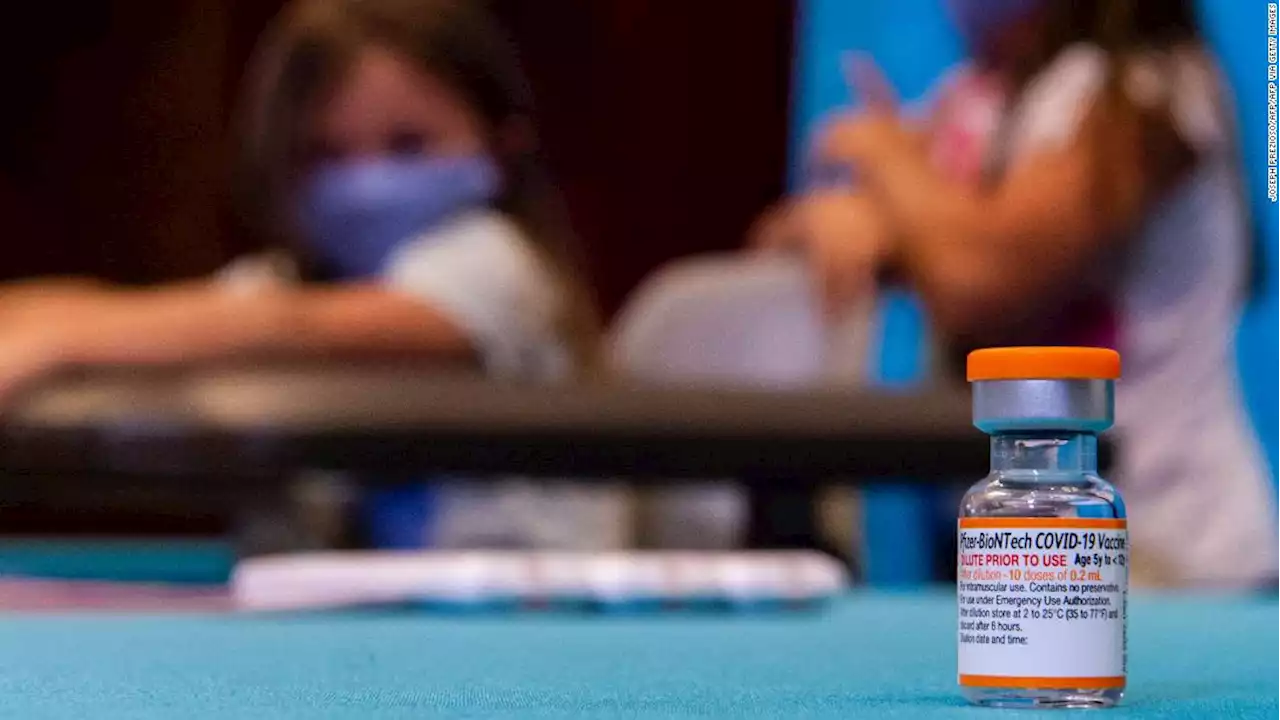 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Read more »
 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Read more »
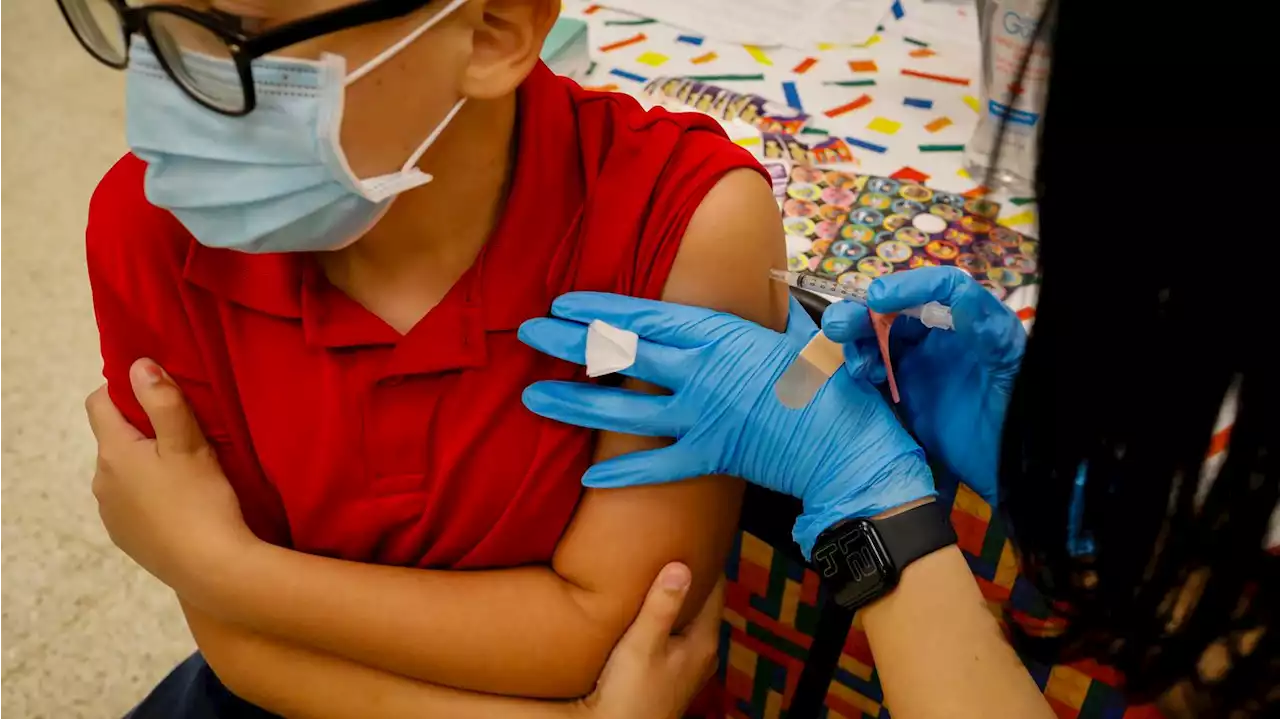 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Read more »
